��Ŀ����
4�� ԭ�������������ӵ�X��Y��Z��W����Ԫ��ԭ��������С��36��Y��̬ԭ�����������������ڲ����������3����X��W��̬ԭ����ͬ����Ԫ����δ�ɶԵ���������ԭ�ӣ�X��Z��ԭ�Ӻ���������֮�͵���Wԭ�Ӻ�����������Z��̬ԭ��ֻ��1��δ�ɶԵĵ��ӣ�
ԭ�������������ӵ�X��Y��Z��W����Ԫ��ԭ��������С��36��Y��̬ԭ�����������������ڲ����������3����X��W��̬ԭ����ͬ����Ԫ����δ�ɶԵ���������ԭ�ӣ�X��Z��ԭ�Ӻ���������֮�͵���Wԭ�Ӻ�����������Z��̬ԭ��ֻ��1��δ�ɶԵĵ��ӣ��ش��������⣺
��1����֪������XZ3��H2Y��Y���⻯�������Ӧ�Ļ�ѧ����ʽΪXZ3+H2Y��XH3+HZY����Ԫ�صĵ縺�ԣ�Y���� Z������ڡ���С�ڡ�����������HZY���ӵĵ���ʽΪ
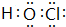 ��
����2��X��һ���⻯�����Է�������Ϊ32��1mol���⻯���еĦҼ�����5NA��
��3��Ԫ��Y��W��һ�ֻ�����ľ���ṹ��ͼ��ʾ����̬Wԭ�ӵĵ����Ų�ʽ��1s22s22p63s23p63d54s1���þ���Ļ�ѧʽΪCr2O3��
��4������ԭ��þ ��ѡ��ء�����þ������ͭ�����γɵľ���Ķѻ���ʽ��������������Y���ӵ�
�ѻ���ʽ��ͬ��
���� ԭ�������������ӵ�X��Y��Z��W����Ԫ��ԭ��������С��36��Y��̬ԭ�����������������ڲ����������3����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����YΪOԪ�أ�X��W��̬ԭ����ͬ����Ԫ����δ�ɶԵ���������ԭ�ӣ�Xԭ����������С����X�ڵڶ����ڣ���Χ�����Ų�Ϊ2s22p3����XΪNԪ�أ���WΪ�������ڵ�P��������ڵ�Cr������X��Z��ԭ�Ӻ���������֮�͵���Wԭ�Ӻ�������������WΪP����ZΪO����Y��ͬ�����Բ��������⣬��WΪCr����ZΪCl������Cl�Ļ�̬ԭ��ֻ��1��δ�ɶԵĵ��ӣ��������⣬�ݴ˽��
��� �⣺ԭ�������������ӵ�X��Y��Z��W����Ԫ��ԭ��������С��36��Y��̬ԭ�����������������ڲ����������3����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����YΪOԪ�أ�X��W��̬ԭ����ͬ����Ԫ����δ�ɶԵ���������ԭ�ӣ�Xԭ����������С����X�ڵڶ����ڣ���Χ�����Ų�Ϊ2s22p3����XΪNԪ�أ���WΪ�������ڵ�P��������ڵ�Cr������X��Z��ԭ�Ӻ���������֮�͵���Wԭ�Ӻ�������������WΪP����ZΪO����Y��ͬ�����Բ��������⣬��WΪCr����ZΪCl������Cl�Ļ�̬ԭ��ֻ��1��δ�ɶԵĵ��ӣ��������⣮
��1����֪������NCl 3��H2O������Ӧ�Ļ�ѧ����ʽΪNCl 3+H2O��NH3+HClO����Ӧ����HClO��OԪ��Ϊ-2�ۣ�ClԪ��Ϊ+1�ۣ���O�ĵõ���������ǿ�����Ե縺��O����Cl��HClO�ĵ���ʽΪ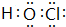 ��
��
�ʴ�Ϊ�����ڣ�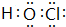 ��
��
��2��NԪ�ص�һ���⻯�����Է�������Ϊ32�����⻯����N2H4����ṹʽΪ �������к���5���Ҽ�������1mol���⻯���еĦҼ�����5NA��
�������к���5���Ҽ�������1mol���⻯���еĦҼ�����5NA��
�ʴ�Ϊ��5NA��
��3��WΪCrԪ�أ�ԭ�Ӻ�����24�����ӣ����Ի�̬ԭ�ӵĵ����Ų�ʽ�ǣ�1s22s22p63s23p63d54s1����ͼ��֪�������ڲ�����4�������ӣ�O2-��ĿΪ��$\frac{1}{2}$��2+$\frac{1}{6}$��12+3=6�����������O2-������֮��Ϊ2��3�����Ըþ���Ļ�ѧʽΪCr2O3��
�ʴ�Ϊ��1s22s22p63s23p63d54s1��Cr2O3��
��4����ͼ��֪���þ���Ϊ�������ܶѻ�����Mg����Ķѻ���ʽ��ͬ��
�ʴ�Ϊ��þ��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų�������ʽ���縺�ԡ���ѧ���������ṹ�����ȣ�ע��ʶ����ѧ���������ṹ������ѻ���ʽ��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д�| A�� | �����ǡ����ǡ���֬�͵�������һ�������¶���ˮ�� | |
| B�� | �������ļ��顢��ϩ��1��3-����ϩ��C4H6���ֱ��ڿ����г��ȼ�գ����������������������� | |
| C�� | �����κ��Լ��������ɼ����Ҵ������ᡢ�����������������л��� | |
| D�� | �����������������ʵ���֮��1��3��ϣ��ڹ����µõ��IJ���ֻ��CHCl3 |
| A�� | 8�����ӵ�̼ԭ�ӣ�12C | B�� | HClO�Ľṹʽ��H-Cl-O | ||
| C�� | þ���ӵĽṹʾ��ͼ�� | D�� | 15N�ĵ����Ų�ʽ��1s22s22p4 |
N2H4��g��+O2��g��=N2��g��+2H2O��g����H1=-533.23kJ•mol-1
H2O��g��=H2O ��l����H2=-44kJ•mol-1
2H2O2��l��=2H2O��l��+O2��g����H3=-196.4kJ•mol-1
��������������ⷴӦ���Ȼ�ѧ����ʽ�ɱ�ʾΪ��������
| A�� | N2H4��g��+2H2O2��l��=N2��g��+4H2O��l����H=+817.63 kJ•mol-1 | |
| B�� | N2H4��g��+2H2O2��l��=N2��g��+4H2O��g����H=-641.63 kJ•mol-1 | |
| C�� | N2H4��g��+2H2O2��l��=N2��g��+4H2O��l����H=-641.63 kJ•mol-1 | |
| D�� | N2H4��g��+2H2O2��l��=N2��g��+4H2O��g����H=-817.63 kJ•mol-1 |
| A�� | ����CO2ͨ��ƫ��������Һ�У�2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32- | |
| B�� | �����������Һ��ͨ������SO2���壺ClO-+SO2+H2O�THClO+HSO${\;}_{3}^{-}$ | |
| C�� | ����������˫��ˮ��KI��Һ��Ӧ��2I-+H2O2+2H+�TI2+2H2O | |
| D�� | ̼�������Һ��������KOH��Һ��Ϻ���ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O |
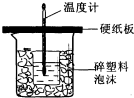 50mL 0.5mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ��㷴Ӧ�ȣ���ش��������⣺
50mL 0.5mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ��㷴Ӧ�ȣ���ش��������⣺ �������л�ѧ�Լ���
�������л�ѧ�Լ���