��Ŀ����
��10�֣�ʵ�������ù����ռ�����480 mL 0��32mol��L��1��NaOH��Һ��
������� g���ռ���壬����Ӧ���� ��������ƽ���̳�����
�����ƹ����У�����Ҫʹ�õ������ǣ�����ţ� ��
A���ձ� B����Ͳ C��������
D��500mL����ƿ E��©��
�۸���ʵ���ʵ����Ҫ�͢����г��������жϣ����ʵ�黹ȱ�ٵ������� �����������ƣ���
�����ڲ����ϵIJ��淶������ʹ������Һ�����ʵ���Ũ��ƫ�͵��� �� ƫ�ߵ��� ��
A������ʱҩƷ������λ�õߵ�
B������ʱ�����
C����Һδ��ȴ��ת������ƿ
D������ƿϴ����δ���
E������ʱ���ӿ̶���
F�����ݺ�תҡ�ȣ�����Һ����ڿ̶��߶�δ��ˮ����
������� g���ռ���壬����Ӧ���� ��������ƽ���̳�����
�����ƹ����У�����Ҫʹ�õ������ǣ�����ţ� ��
A���ձ� B����Ͳ C��������
D��500mL����ƿ E��©��
�۸���ʵ���ʵ����Ҫ�͢����г��������жϣ����ʵ�黹ȱ�ٵ������� �����������ƣ���
�����ڲ����ϵIJ��淶������ʹ������Һ�����ʵ���Ũ��ƫ�͵��� �� ƫ�ߵ��� ��
A������ʱҩƷ������λ�õߵ�
B������ʱ�����
C����Һδ��ȴ��ת������ƿ
D������ƿϴ����δ���
E������ʱ���ӿ̶���
F�����ݺ�תҡ�ȣ�����Һ����ڿ̶��߶�δ��ˮ����
��1��6.4��С�ձ���2��E ��3����ͷ�ιܣ�4��AB��CE
��1����������ƿû��480ml���ģ�����Ӧ������500ml������Ҫ�������Ƶ�������0.5L��0.32mol/L��40g/mol��6.4g������ʱ��������Ӧ�÷���С�ձ��У�Խǿ������������ˮ��ʴ���̡�
��2��ת����Һ��Ҫ������������Ҫ©�������Դ�ѡE��
��3������������������֪����ȱ�ٶ���ʱ�Ľ�ͷ�ιܡ�
��4������c��n/V��֪��A���Լ����������٣�Ũ��ƫ�ͣ�B������������ˮ�����ʼ��٣�Ũ��ƫ�ͣ�C�и�������������֪����Һ�����ƫ�٣�Ũ��ƫ�ߣ�����ƿ����Ҫ��ɣ�D��Ӱ�죻E����Һ�����ƫ�٣�Ũ��ƫ�ߣ�F��Ӱ�졣
��2��ת����Һ��Ҫ������������Ҫ©�������Դ�ѡE��
��3������������������֪����ȱ�ٶ���ʱ�Ľ�ͷ�ιܡ�
��4������c��n/V��֪��A���Լ����������٣�Ũ��ƫ�ͣ�B������������ˮ�����ʼ��٣�Ũ��ƫ�ͣ�C�и�������������֪����Һ�����ƫ�٣�Ũ��ƫ�ߣ�����ƿ����Ҫ��ɣ�D��Ӱ�죻E����Һ�����ƫ�٣�Ũ��ƫ�ߣ�F��Ӱ�졣

��ϰ��ϵ�д�
�����Ŀ







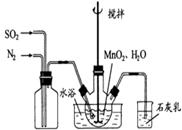
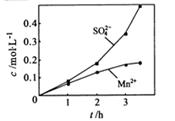
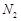 ���ɿ�������÷�ӦҺ��Mn2+��SO42-��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��Mn2+��SO42-Ũ�ȱ仯�������Բ����ԭ���� ��
���ɿ�������÷�ӦҺ��Mn2+��SO42-��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��Mn2+��SO42-Ũ�ȱ仯�������Բ����ԭ���� ��