��Ŀ����
��ͳ������ըҩ�������к�Pb��ʹ��ʱ��������Ⱦ��ͬʱ���������ʣ��ըҩ������Σ���Ӵ��ߵ�������ȫ������UNC��ѧ����Thomas J��Meyer���з��˻����Ѻá���ȫ�͵ġ���ɫ������ըҩ������һ�ֿɱ�ʾΪNa2R����������ˮ�п���ʧȥ���ԣ���ը�����Σ���Բ������֪10mL Na2R��Һ��Na+������ΪN������Na2R��Һ�����ʵ���Ũ��Ϊ
- A.N��10-2 mol/L
- B.
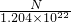 mol/L
mol/L - C.
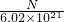 mol/L
mol/L - D.
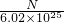 mol/L
mol/L
B
����������n= ����Na+�����ʵ�����n��Na2R��=
����Na+�����ʵ�����n��Na2R��=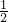 n��Na+��������c=
n��Na+��������c=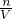 �����Na2R��Һ�����ʵ���Ũ�ȣ�
�����Na2R��Һ�����ʵ���Ũ�ȣ�
���N��Na+�����ʵ���Ϊ =
= mol��
mol��
����n��Na2R��=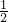 n��Na+��=
n��Na+��=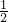 ��
�� mol=
mol= mol��
mol��
��Na2R��Һ�����ʵ���Ũ��Ϊ =
=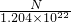 mol/L��
mol/L��
��ѡB��
���������⿼�����ʵ���Ũ�ȵ��йؼ��㣬�ѶȲ���ע����������Ũ��������Ũ�ȵĹ�ϵ��
����������n=
 ����Na+�����ʵ�����n��Na2R��=
����Na+�����ʵ�����n��Na2R��=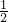 n��Na+��������c=
n��Na+��������c=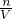 �����Na2R��Һ�����ʵ���Ũ�ȣ�
�����Na2R��Һ�����ʵ���Ũ�ȣ����N��Na+�����ʵ���Ϊ
 =
= mol��
mol������n��Na2R��=
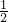 n��Na+��=
n��Na+��=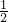 ��
�� mol=
mol= mol��
mol����Na2R��Һ�����ʵ���Ũ��Ϊ
 =
=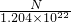 mol/L��
mol/L����ѡB��
���������⿼�����ʵ���Ũ�ȵ��йؼ��㣬�ѶȲ���ע����������Ũ��������Ũ�ȵĹ�ϵ��

��ϰ��ϵ�д�
�����Ŀ
 mol/L
mol/L mol/L
mol/L mol/L
mol/L mol/L
mol/L mol/L
mol/L mol/L
mol/L mol/L
mol/L mol/L
mol/L mol/L
mol/L