��Ŀ����
ʵ�����ù����ռ�����450mL0.25mol��L��1��CuSO4��Һ��
��1�������_____________g��CuSO4��5H2O���塣
��2�����ƹ����У�����Ҫʹ�õ�������(�����)_____________��
���ձ�����Ͳ�۲�������1000mL����ƿ��©����500mL�Լ�ƿ
��3������ʵ���ʵ����Ҫ�ͣ�2�����г��������жϣ����ʵ�黹ȱ�ٵIJ���������__________________ (����������)��
��4�����в���ʹ���Ƴ�����ҺŨ��ƫ�͵��ǣ����ţ�____________��
A������ҩƷʱ����ŷ� |
B������Һת�Ƶ�����ƿʱ����Һ���� |
C��ת����Һǰ����ƿ��������ˮ |
D������ʱ���Ӷ��� |
E������ʱ���Ӷ���
F�����ݺ�������ƿ��Һ�峬�����ߣ��ý�ͷ�ι�����һ����
��ϰ��ϵ�д�
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
�����Ŀ



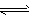 2NH3���ù����л�ԭ��Ӧ�ķ���ʽΪ________________��
2NH3���ù����л�ԭ��Ӧ�ķ���ʽΪ________________��
 2FeCl2+CuCl2��FeCl3 ��ҺҲ��������Ӧ2FeCl3+Fe
2FeCl2+CuCl2��FeCl3 ��ҺҲ��������Ӧ2FeCl3+Fe A����ʹpH��ֽ������ɫ����Һ�У�Na+��NH4+��NO3-��Cl-
A����ʹpH��ֽ������ɫ����Һ�У�Na+��NH4+��NO3-��Cl-