��Ŀ����
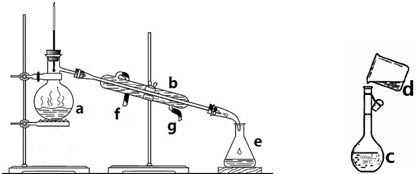
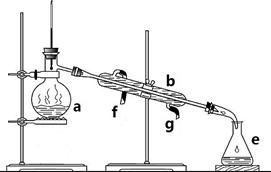
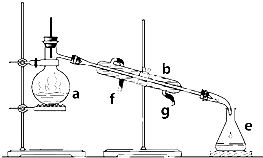 �����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
�����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã���1��д���������������ƣ�a��
������ƿ
������ƿ
��b������
������
��e����ƿ
��ƿ
����2���������Ӻ�ʹ��ǰ������еIJ�����
���װ��������
���װ��������
����3��������װ�â�������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ�������
�ƾ���
�ƾ���
���������������������ʵ�飬�¶ȼ�ˮ�����λ����������ƿ֧�ܿ�
������ƿ֧�ܿ�
��������ˮ��g
g
����f��g����ͨ�룬f
f
����������������1������װ���е���Ҫ������������ƿ�������ܡ�ţ�ǹܡ���ƿ���ƾ��ƣ����������Ľṹ�ص��жϣ�
��2������ʱ�������壬Ϊ��ֹ©����Ӧ�ȼ��������ԣ�
��3���������Ȼ�̼�;ƾ��Ļ��������þƾ��Ƽ��ȣ�ʵ����������̣��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ��ݴ˼��ɽ��
��2������ʱ�������壬Ϊ��ֹ©����Ӧ�ȼ��������ԣ�
��3���������Ȼ�̼�;ƾ��Ļ��������þƾ��Ƽ��ȣ�ʵ����������̣��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ��ݴ˼��ɽ��
����⣺��1������װ���е���Ҫ������������ƿ�������ܡ�ţ�ǹܡ���ƿ���ƾ��ƣ�aΪ������ƿ��bΪ�����ܣ�eΪ��ƿ��
�ʴ�Ϊ��������ƿ�������ܣ���ƿ��
��2�������Ƿ�����ᴿ�е㲻ͬ��Һ̬����ﳣ�õķ���֮һ������Һ�������и���ַе�IJ�ͬ��ʹҺ�����ﲿ����������֮ʹ���������������Ӷ�ʵ����������ֵķ��룬������в������壬���������Ӻ�ʹ��ǰ������еIJ��������������ԣ�
�ʴ�Ϊ�����װ�õ������ԣ�
��3���������Ȼ�̼�;ƾ��Ļ����������ķ������룬�����þƾ��ƣ��¶ȼ�Ӧλ��������ƿ֧�ܿڣ��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
�ʴ�Ϊ���ƾ��ƣ�������ƿ֧�ܿڣ� g��f��
�ʴ�Ϊ��������ƿ�������ܣ���ƿ��
��2�������Ƿ�����ᴿ�е㲻ͬ��Һ̬����ﳣ�õķ���֮һ������Һ�������и���ַе�IJ�ͬ��ʹҺ�����ﲿ����������֮ʹ���������������Ӷ�ʵ����������ֵķ��룬������в������壬���������Ӻ�ʹ��ǰ������еIJ��������������ԣ�
�ʴ�Ϊ�����װ�õ������ԣ�
��3���������Ȼ�̼�;ƾ��Ļ����������ķ������룬�����þƾ��ƣ��¶ȼ�Ӧλ��������ƿ֧�ܿڣ��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
�ʴ�Ϊ���ƾ��ƣ�������ƿ֧�ܿڣ� g��f��
���������⿼�黯ѧʵ��Ļ�������֪ʶ�����������ԭ�������ݲ����������ǽ���Ĺؼ�����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ