��Ŀ����
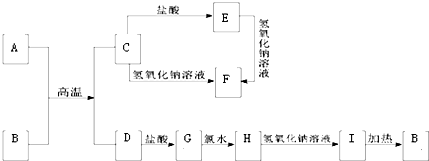
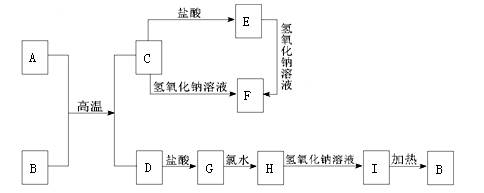
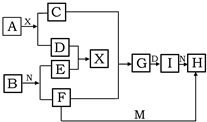 ��������ѧ��ѧ�г������ʵ�ת����ϵͼ�У���֪������AΪ����ɫ���廯���BΪ�������ʣ�D��E��M�dz������嵥�ʣ�����IΪ���ɫ���壬MΪ����ɫ��N��θ�����Ҫ�ɷ֣���ҵ����E��M����ȡN�����ƶϣ�
��������ѧ��ѧ�г������ʵ�ת����ϵͼ�У���֪������AΪ����ɫ���廯���BΪ�������ʣ�D��E��M�dz������嵥�ʣ�����IΪ���ɫ���壬MΪ����ɫ��N��θ�����Ҫ�ɷ֣���ҵ����E��M����ȡN�����ƶϣ���1��д�����л�ѧʽ A
Na2O2
Na2O2
IFe��OH��3
Fe��OH��3
��2��Gת��ΪI������Ϊ
��ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ
��ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ
��3��F+M��H���ӷ���ʽΪ
2Fe2++Cl2=2Fe3++2Cl-
2Fe2++Cl2=2Fe3++2Cl-
��4��A+X��C+D�Ļ�ѧ����ʽΪ
2Na2O2+2H2O=4NaOH+O2��
2Na2O2+2H2O=4NaOH+O2��
����������֪������AΪ����ɫ���廯�����ж�ΪNa2O2��BΪ�������ʣ�D��E��M�dz������嵥�ʣ�����IΪ���ɫ�����ж�ΪFe��OH��3��MΪ����ɫΪCl2��N��θ�����Ҫ�ɷ��ƶ�ΪHCl����ҵ����E��M����ȡN��˵��EΪH2������ת����ϵ�еķ�Ӧ��֪��CΪNaOH��DΪO2��XΪH2O�����ƶϳ�I�жϣ�GΪFe��OH��2��FΪFeCl2��ȷ��BΪFe�������жϽ�������ش����⣻
�����֪������AΪ����ɫ���廯�����ж�ΪNa2O2��BΪ�������ʣ�D��E��M�dz������嵥�ʣ�����IΪ���ɫ�����ж�ΪFe��OH��3��MΪ����ɫΪCl2��N��θ�����Ҫ�ɷ��ƶ�ΪHCl��HΪFeCl3����ҵ����E��M����ȡN��˵��EΪH2������ת����ϵ�еķ�Ӧ��֪��CΪNaOH��DΪO2��XΪH2O�����ƶϳ�I�жϣ�GΪFe��OH��2��FΪFeCl2��ȷ��BΪFe��
��1�����ݷ����ж�AΪ��Na2O2 �� I�Ļ�ѧʽΪ��Fe��OH��3���ʴ�Ϊ��Na2O2 ��Fe��OH��3��
��2��G��Fe��OH��2��ת��ΪI��Fe��OH��3���������ǣ���ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ���ʴ�Ϊ����ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ��
��3��F��FeCl2��+M��Cl2����H��FeCl3������Ӧ�����ӷ���ʽΪ��2Fe2++Cl2=2Fe3++2Cl-���ʴ�Ϊ��2Fe2++Cl2=2Fe3++2Cl-��
��4��A��Na2O2��+X��H2O����C��NaOH��+D��O2������Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
��1�����ݷ����ж�AΪ��Na2O2 �� I�Ļ�ѧʽΪ��Fe��OH��3���ʴ�Ϊ��Na2O2 ��Fe��OH��3��
��2��G��Fe��OH��2��ת��ΪI��Fe��OH��3���������ǣ���ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ���ʴ�Ϊ����ɫ����Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ��
��3��F��FeCl2��+M��Cl2����H��FeCl3������Ӧ�����ӷ���ʽΪ��2Fe2++Cl2=2Fe3++2Cl-���ʴ�Ϊ��2Fe2++Cl2=2Fe3++2Cl-��
��4��A��Na2O2��+X��H2O����C��NaOH��+D��O2������Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
���������⿼��������ת����ϵ������Ӧ�ã��������ʵ��ۺ�Ӧ�ã���Ҫ�����Ƽ��仯��������仯�������ʵ�Ӧ�ã����ӷ���ʽ����д��������������ķ���Ӧ���ǽ���ؼ���

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ