��Ŀ����
��17�֣��״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ������ҵ��һ��ɲ������·�Ӧ���ϳɼ״��� CO(g)+2H2(g) 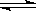 CH3OH(g)��
CH3OH(g)��
��1�������÷�Ӧ���ش��������⣺
��ƽ�ⳣ������ʽΪK= ��
�����и����У����ܹ�˵���÷�Ӧ�Ѵﵽƽ�����______________������ţ���
a. ���¡����������£������ڵ�ѹǿ�������仯
b. һ�������£�CH3OH�ֽ�����ʺ�CH3OH���ɵ��������
c. һ�������£�CO��H2��CH3OH��Ũ�ȱ��ֲ���
d. һ�������£���λʱ��������2 mol CO��ͬʱ����1 mol CH3OH
��2����ͼ�Ǹ÷�Ӧ�ڲ�ͬ�¶���CO��ת������ʱ��仯�����ߡ�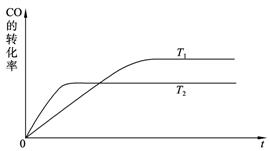
�ٸ÷�Ӧ���ʱ���H____________0(�����������������)��
��T1��T2�¶��µ�ƽ�ⳣ����С��ϵ��K1____________K2(�����������������)��
���������ݻ����䣬���д�ʩ�����Ӽ״����ʵ���______________��
a. �����¶�b. ��CH3OH(g)����ϵ�з���
c. ʹ�ú��� �Ĵ���d. ����He��ʹ��ϵ��ѹǿ����
�Ĵ���d. ����He��ʹ��ϵ��ѹǿ����
��3����֪�ڳ��³�ѹ�£�
�� 2CH3OH(l)+3O2(g)=2CO2(g)+4H2O(g)��H��-a kJ��mol-1
�� 2CO(g)+O2(g)=2CO2(g) ��H��-b kJ��mol-1
�� H2O(g)= H2O(l) ��H��-c kJ��mol-1
��CH3OH(l)+O2(g) =CO(g)+2H2O(l) ��H��______________kJ��mol-1��
��4�� 2009��10�£��й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѡ��״�ȼ�ϵ�صĹ���ԭ����ͼ��ʾ��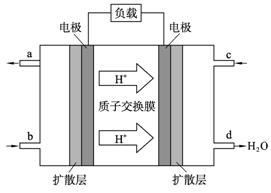
�ٸõ�ع���ʱ��b��ͨ�������Ϊ____________, c��ͨ�������Ϊ__________��
�ڸõ�������ĵ缫��ӦʽΪ:_______________________________��
�۹���һ��ʱ���6.4 g�״���ȫ��Ӧ����CO2ʱ����___________NA������ת�ơ�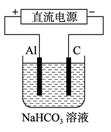
��5���������������Դ������ͼ��ʾװ�ã���ʵ������ģ������Ʒ���桰�ۻ��������Ĺ����У�������Һ����ǣ�ԭ���ǣ�����صĵ缫��Ӧʽ�����ӷ���ʽ��ʾ����
_______________________________________________________��
_______________________________________________________��
��17�֣�
��1����K=��CH3OH��/��CO�ݣ�H2��2(1��);�� d��1�֣�
��2���� <��1�֣��� >��1�֣���b��1�֣�
��3����b-a-4c��/2��3�֣�
��4���� CH3OH��1�֣�;O2��1�֣��� O2+4e-+4H+��2H2O ��2�֣�;�� 1.2��2�֣�
��2�֣�;�� 1.2��2�֣�
��5�� Al��Al3++3e-��1�֣�Al3++3HCO3- =Al(OH)3��+3CO2����2�֣�
����

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д�
 ��Դ��ȱ���������ٵ��ش����⣮�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
��Դ��ȱ���������ٵ��ش����⣮�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ���� ��2012?������һģ����Դ��ȱ������������ٵ��ش����⣮�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
��2012?������һģ����Դ��ȱ������������ٵ��ش����⣮�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����