��Ŀ����
���õ���ȩˮ��Һ������ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ���ݲⶨ���ϲ�����Ϊ��ȩ�Ļ�״�ۺ���(C2H4O)n�����ķе��ˮ�ķе�ߣ���������ȩ������ȩ�ķе���20.8 �棬������Һ���ױ���������������ķе���117.9 �棬�Ӿ��õ���ȩˮ��Һ����ȡ��ȩ(�Եõ���ȩˮ��Һ)�����������·�Ӧ��(C2H4O)n�Իش��������⣺
(1)�ȷ�������õ�(C2H4O)n�����������ǣ������������Һ©���У����÷ֲ���������²�Һ������ձ��У�Ȼ��___________________��
(2)���һ��ʵ��֤�����õ���ȩ�Ƿ�����(д����Ҫ�������衢ʹ�õ��Լ���ʵ������ͽ���)��____________________________��
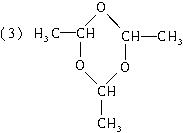
(3)��n��3ʱ����д��(C2H4O)n�Ľṹ��ʽ_________________��
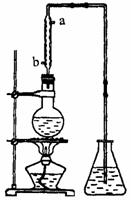
(4)��ȡ��ȩ��װ������ͼ����ƿ�е�Һ����(C2H4O)n��6 mol��L-1�Ļ�����ƿ��ʢ������ˮ�����Ȼ���������ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ�С�
��������������ˮ�Ľ�����_________(�a����b��)��
����ʵ������в�ʹ����������ȴ�����������Ľ��У���Һ���к�ɫ���ʺʹ̼�����ζ�������ɡ����û�ѧ����ʽ��ʾ��һ����_____________________________________��
�۵���ƿ�ڵ��ܿ�����Խ��Խ��ʱ��������ȩ��������������ʵ����������ʵ��װ�õĵ�һ��������___________________________��
(1)���ϲ����״Һ��(C2H4O)n�ӷ�Һ©�����Ͽڵ���
(2)ȡ�����²�ˮ��Һ���μ�ʯ��ָʾ��������Һ�Ժ�ɫ����˵��������ȩ������(���������𰸾���)

(4)��b��
��CH3CHO+H2SO4![]() 2C+SO2��+3H2O��
2C+SO2��+3H2O��
���ȳ������ܣ����ⷢ������

| |||||||||||||||||||
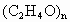 �����ķе��ˮ�ķе�ߣ���������ȩ������ȩ�ķе���20.8�棬������Һ���ױ���������������ķе���117.9�棬�Ӿ��õ���ȩˮ��Һ����ȡ��ȩ(�Եõ���ȩˮ��Һ)���練Ӧ�˴���ͼ������
�����ķе��ˮ�ķе�ߣ���������ȩ������ȩ�ķе���20.8�棬������Һ���ױ���������������ķе���117.9�棬�Ӿ��õ���ȩˮ��Һ����ȡ��ȩ(�Եõ���ȩˮ��Һ)���練Ӧ�˴���ͼ������ �Ļ�����ƿ��ʢ������ˮ�����Ȼ���������ڣ�
�Ļ�����ƿ��ʢ������ˮ�����Ȼ���������ڣ�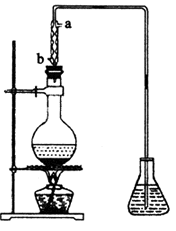

 ��3����n��3ʱ����д��(C2H4O)n�Ľṹ��ʽ__________________��
��3����n��3ʱ����д��(C2H4O)n�Ľṹ��ʽ__________________��