��Ŀ����
�ɵ����ʵ�����NaHCO3��KHCO3��ɵĻ����18.400g����100mL���ᷴӦ���������漰�����������Ϊ��״���£����ʱ�����ô���ĸ��ʽ�ӱ�ʾ����
��1���û������NaHCO3��KHCO3�������ֱ�Ϊ______��______��
��2����̼������������ǡ����ȫ��Ӧ������������ʵ���Ũ��Ϊ______mol?L-1��
��3������������������CO2�����Ϊ______ L��
��4�������Ӧ��̼��������ʣ�࣬���������Ҫ��������CO2�����������Ҫ֪��______��
��5����NaHCO3��KHCO3�����Ե����ʵ�����ϣ���18.4g����������������������ȫ��Ӧʱ����CO2�����ʵ�������n��ʾ���ķ�Χ��______��
�⣺��1����NaHCO3��KHCO3�����ʵ���Ϊnmol����������֮��Ϊ18.4g����
84g/mol��nmol+100g/mol��nmol=18.4�����n=0.1��
����NaHCO3������Ϊ84g/mol��0.1mol=8.4g��KHCO3������Ϊ100g/mol��0.1mol=10g��
�ʴ�Ϊ��8.4��10��
��2��NaHCO3��KHCO3��ɵĻ������100mL����ǡ�÷�Ӧ��̼�����������ᰴ1��1��Ӧ����n��HCl��=n��NaHCO3��+��KHCO3�����ɣ�1��֪n��NaHCO3��=��KHCO3��=0.1mol��n��HCl��=n��NaHCO3��+��KHCO3��=0.1mol+0.1mol=0.2mol��������������ʵ���Ũ��Ϊ =2mol/L��
=2mol/L��
�ʴ�Ϊ��2��
��3�����������NaHCO3��KHCO3��ɵĻ������ȫ��Ӧ������̼Ԫ���غ��֪��n��CO2��=n��NaHCO3��+��KHCO3��=0.1mol+0.1mol=0.2mol�����Ա�״��������CO2�����Ϊ0.2mol��22.4L/mol=4.48L��
�ʴ�Ϊ��4.48��
��4��̼�����������ᰴ1��1��Ӧ�����������̼��������ʣ�࣬Ӧ����HCl�����ʵ������������̼�����������Ҫ��������CO2�����������Ҫ֪����������ʵ���Ũ�ȣ�
�ʴ�Ϊ����������ʵ���Ũ�ȣ�
��5���ٶ�̼������ȫ��NaHCO3����n��NaHCO3��= =0.219mol��NaHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��NaHCO3��=0.219mol���ٶ�̼������ȫ��KHCO3����n��KHCO3��=
=0.219mol��NaHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��NaHCO3��=0.219mol���ٶ�̼������ȫ��KHCO3����n��KHCO3��= =0.184mol��KHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��KHCO3��=0.184mol������ʵ�ʶ�����̼�����ʵ���Ϊ0.184mol��n��CO2����0.219mol��
=0.184mol��KHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��KHCO3��=0.184mol������ʵ�ʶ�����̼�����ʵ���Ϊ0.184mol��n��CO2����0.219mol��
�ʴ�Ϊ��0.184mol��n��0.219mol��
��������1����NaHCO3��KHCO3�����ʵ���Ϊnmol�����ݶ�������֮��Ϊ18.4g���з��̼���n��ֵ���ڸ���m=nM������߸��Ե�������
��2��NaHCO3��KHCO3��ɵĻ������100mL����ǡ�÷�Ӧ����n��HCl��=n��NaHCO3��+��KHCO3�����ٸ���c=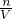 ����Ԫ�ص�Ũ�ȣ�
����Ԫ�ص�Ũ�ȣ�
��3������̼Ԫ���غ��֪��n��CO2��=n��NaHCO3��+��KHCO3�����ٸ���V=nVm�������ɵĶ�����̼�������
��4��̼�����������ᰴ1��1��Ӧ�����������̼��������ʣ�࣬Ӧ����HCl�����ʵ������������̼�������
��5���ٶ�̼������ȫ��NaHCO3������̼Ԫ���غ�������ɶ�����̼�����ʵ������ٶ�̼������ȫ��KHCO3������̼Ԫ���غ�������ɶ�����̼�����ʵ�����ʵ�ʶ������ɶ�����̼�����ʵ���֮�䣮
���������⿼������ļ��㡢���ݷ���ʽ�ļ���ȣ��Ѷ��еȣ���5���з�Χ�ļ��㣬ע�⼫�������ã�
84g/mol��nmol+100g/mol��nmol=18.4�����n=0.1��
����NaHCO3������Ϊ84g/mol��0.1mol=8.4g��KHCO3������Ϊ100g/mol��0.1mol=10g��
�ʴ�Ϊ��8.4��10��
��2��NaHCO3��KHCO3��ɵĻ������100mL����ǡ�÷�Ӧ��̼�����������ᰴ1��1��Ӧ����n��HCl��=n��NaHCO3��+��KHCO3�����ɣ�1��֪n��NaHCO3��=��KHCO3��=0.1mol��n��HCl��=n��NaHCO3��+��KHCO3��=0.1mol+0.1mol=0.2mol��������������ʵ���Ũ��Ϊ
 =2mol/L��
=2mol/L���ʴ�Ϊ��2��
��3�����������NaHCO3��KHCO3��ɵĻ������ȫ��Ӧ������̼Ԫ���غ��֪��n��CO2��=n��NaHCO3��+��KHCO3��=0.1mol+0.1mol=0.2mol�����Ա�״��������CO2�����Ϊ0.2mol��22.4L/mol=4.48L��
�ʴ�Ϊ��4.48��
��4��̼�����������ᰴ1��1��Ӧ�����������̼��������ʣ�࣬Ӧ����HCl�����ʵ������������̼�����������Ҫ��������CO2�����������Ҫ֪����������ʵ���Ũ�ȣ�
�ʴ�Ϊ����������ʵ���Ũ�ȣ�
��5���ٶ�̼������ȫ��NaHCO3����n��NaHCO3��=
 =0.219mol��NaHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��NaHCO3��=0.219mol���ٶ�̼������ȫ��KHCO3����n��KHCO3��=
=0.219mol��NaHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��NaHCO3��=0.219mol���ٶ�̼������ȫ��KHCO3����n��KHCO3��= =0.184mol��KHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��KHCO3��=0.184mol������ʵ�ʶ�����̼�����ʵ���Ϊ0.184mol��n��CO2����0.219mol��
=0.184mol��KHCO3��ȫ��Ӧ������̼Ԫ���غ㣬��֪���ɶ�����̼n��CO2��=n��KHCO3��=0.184mol������ʵ�ʶ�����̼�����ʵ���Ϊ0.184mol��n��CO2����0.219mol���ʴ�Ϊ��0.184mol��n��0.219mol��
��������1����NaHCO3��KHCO3�����ʵ���Ϊnmol�����ݶ�������֮��Ϊ18.4g���з��̼���n��ֵ���ڸ���m=nM������߸��Ե�������
��2��NaHCO3��KHCO3��ɵĻ������100mL����ǡ�÷�Ӧ����n��HCl��=n��NaHCO3��+��KHCO3�����ٸ���c=
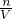 ����Ԫ�ص�Ũ�ȣ�
����Ԫ�ص�Ũ�ȣ���3������̼Ԫ���غ��֪��n��CO2��=n��NaHCO3��+��KHCO3�����ٸ���V=nVm�������ɵĶ�����̼�������
��4��̼�����������ᰴ1��1��Ӧ�����������̼��������ʣ�࣬Ӧ����HCl�����ʵ������������̼�������
��5���ٶ�̼������ȫ��NaHCO3������̼Ԫ���غ�������ɶ�����̼�����ʵ������ٶ�̼������ȫ��KHCO3������̼Ԫ���غ�������ɶ�����̼�����ʵ�����ʵ�ʶ������ɶ�����̼�����ʵ���֮�䣮
���������⿼������ļ��㡢���ݷ���ʽ�ļ���ȣ��Ѷ��еȣ���5���з�Χ�ļ��㣬ע�⼫�������ã�

��ϰ��ϵ�д�
�����Ŀ


 �¡�
�¡�
 �ġ�
�ġ�