��Ŀ����
��1�������������ڷŵ�ʱ���ܷ�Ӧ����ʽΪ��Fe+NiO2+2H2O=Fe(OH)2+Ni(OH)2
�õ�صĵ������ҺӦΪ�� ��ѡ�����ԡ����ԡ�����Һ
������ӦʽΪ�� ������ӦʽΪ��
��2����������ѧ��ѧʵ���г����ļ��ֶ���������
a����Ͳ b������ƿ c��������ƽ d���¶ȼ�
�����б�ʾ������ʹ���¶ȵ���_____________(��д���)��
��ʹ��ǰҪ��������Ƿ�©Һ����____________________(��д���)��
�۳�ȡ10.5g������Ʒ(1g����ʹ������)ʱ������Ʒ��������ƽ�����̣���������Ʒ��ʵ������Ϊ_______________g��
�õ�صĵ������ҺӦΪ�� ��ѡ�����ԡ����ԡ�����Һ
������ӦʽΪ�� ������ӦʽΪ��
��2����������ѧ��ѧʵ���г����ļ��ֶ���������
a����Ͳ b������ƿ c��������ƽ d���¶ȼ�
�����б�ʾ������ʹ���¶ȵ���_____________(��д���)��
��ʹ��ǰҪ��������Ƿ�©Һ����____________________(��д���)��
�۳�ȡ10.5g������Ʒ(1g����ʹ������)ʱ������Ʒ��������ƽ�����̣���������Ʒ��ʵ������Ϊ_______________g��
��1�����ԣ������� NiO2+2e��+2H2O = Ni(OH)2 +2OH��
������Fe��2e��+ 2OH��= Fe(OH)2
��2���� ab �� b ��9.5 ��ÿ��3�֣���18�֣�
������Fe��2e��+ 2OH��= Fe(OH)2
��2���� ab �� b ��9.5 ��ÿ��3�֣���18�֣�
��

��ϰ��ϵ�д�
�����Ŀ
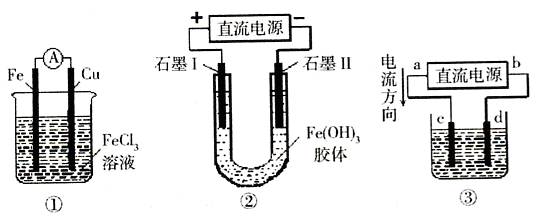

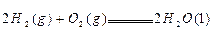 ��
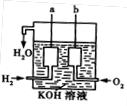
��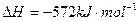 ,
, 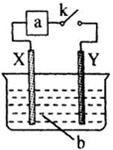
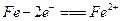
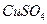 ��Һʱ��X��Y���缫������
��Һʱ��X��Y���缫������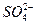 Ũ�����
Ũ�����