��Ŀ����
19���ɽ�������PCL�Ľṹ�ɱ�ʾΪ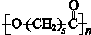 �� ��ϳ�·�����£�
�� ��ϳ�·�����£�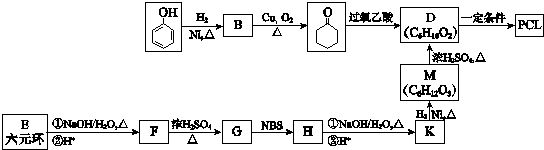
��֪��
��
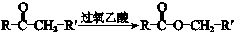
��R-CH=CH-CH3$\stackrel{NBS}{��}$R-CH=CH-CH3Br
�ش��������⣺
��1��B�����������ŵ��������ǻ���
��2��D�Ľṹ��ʽ��
 ��
����3��M�ķ��ӽṹ����֧����M����D�ķ�Ӧ������������Ӧ��ȡ����Ӧ����
��4��E��D��ͬ���칹�壬������ͬ�Ĺ����ţ�E�Ľṹ��ʽ��
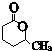 ��
����5������˵����ȷ����bc��
a��Kû��˳���칹 b��M��һ�������¿ɷ����ۺϷ�Ӧ c��PCL�Ľṹ�к�������
��6��H����������������Һ��Ӧ�Ļ�ѧ����ʽ��HOOCCH2CH2CH=CHCH2Br+2NaOH$��_{��}^{ˮ}$NaOOCCH2CH2CH=CHCH2OH+NaBr+H2O��
��7��M���������Z�����Ǻϳ�������ԭ��֮һ����B��ԭ�Ͽ��Ƶü����ᣬ���������Ϣ�����ϳ�·������ͼ�����Լ���ѡ����
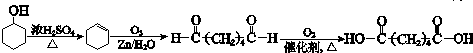 ��
����֪��
 �ϳ�·������ͼʾ����
�ϳ�·������ͼʾ����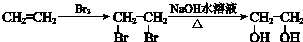
���� �����������ӳ����ɻ�����B��BΪ ����������Cu���������������������ɻ���ͪ�������Ϣ�ٻ���ͪ��������ᷴӦ����D��DΪ
����������Cu���������������������ɻ���ͪ�������Ϣ�ٻ���ͪ��������ᷴӦ����D��DΪ ��M�ķ��ӽṹ����֧������MΪHOOC��CH2��5OH��M�ܹ�ͨ��������Ӧ���������ɸ߷��ӻ����E��D��ͬ���칹�壬������ͬ�Ĺ����ţ���֪EΪ
��M�ķ��ӽṹ����֧������MΪHOOC��CH2��5OH��M�ܹ�ͨ��������Ӧ���������ɸ߷��ӻ����E��D��ͬ���칹�壬������ͬ�Ĺ����ţ���֪EΪ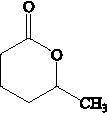 ��Eˮ������F��FΪHOOC��CH2��3CHOHCH3��F��GΪ��ȥ��Ӧ�������Ϣ�ں�G��H�ķ�Ӧ������֪��GΪHOOCCH2CH2CH=CHCH3��HΪHOOCCH2CH2CH=CHCH2Br��Hˮ������K��KΪHOOCCH2CH2CH=CHCH2OH����̼̼˫�����������Ų�ͬ������˳���칹���ݴ˷�����
��Eˮ������F��FΪHOOC��CH2��3CHOHCH3��F��GΪ��ȥ��Ӧ�������Ϣ�ں�G��H�ķ�Ӧ������֪��GΪHOOCCH2CH2CH=CHCH3��HΪHOOCCH2CH2CH=CHCH2Br��Hˮ������K��KΪHOOCCH2CH2CH=CHCH2OH����̼̼˫�����������Ų�ͬ������˳���칹���ݴ˷�����
��� �⣺��1�������������ӳ����ɻ��������������к����ǻ����ʴ�Ϊ���ǻ���
��2����������Cu���������������������ɻ���ͪ�������Ϣ��DΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3����MΪHOOC��CH2��5OH��DΪ ��Mͨ��������Ӧ����D���ʴ�Ϊ��������Ӧ��ȡ����Ӧ����
��Mͨ��������Ӧ����D���ʴ�Ϊ��������Ӧ��ȡ����Ӧ����
��4��E��D��ͬ���칹�壬������ͬ�Ĺ����ţ���֪EΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5��a��KΪHOOCCH2CH2CH=CHCH2OH��K��̼̼˫��������ԭ���Ų�ͬ������˳���칹����a����
b��MΪHOOC��CH2��5OH���ܹ�ͨ��������Ӧ�ķ�ʽ�������ɸ߷��ӻ������b��ȷ��
c��PCL�Ľṹ�ɱ�ʾ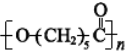 ��������֮��ͨ��������������c��ȷ��
��������֮��ͨ��������������c��ȷ��
�ʴ�Ϊ��bc��
��6��HΪHOOCCH2CH2CH=CHCH2Br����NaOH��Һ�з����кͷ�Ӧ��±������ˮ�ⷴӦ����ѧ����ʽΪHOOCCH2CH2CH=CHCH2Br+2NaOH$��_{��}^{ˮ}$NaOOCCH2CH2CH=CHCH2OH+NaBr+H2O���ʴ�Ϊ��HOOCCH2CH2CH=CHCH2Br+2NaOH$��_{��}^{ˮ}$NaOOCCH2CH2CH=CHCH2OH+NaBr+H2O��
��7��BΪ ��������������ȥ��Ӧ���ɻ���ϩ����������Ϣ���ó�����������OHCCH2CH2CH2CH2CHO�����������ɼ����ᣬ�ϳ�·��Ϊ��
��������������ȥ��Ӧ���ɻ���ϩ����������Ϣ���ó�����������OHCCH2CH2CH2CH2CHO�����������ɼ����ᣬ�ϳ�·��Ϊ��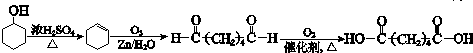 ��
��
���� ���⿼���л���ĺϳɣ���Ŀ�Ѷ��еȣ������ע���������Ϣ�����ŵ�ת�����ر����л�������ŵ����ʣ��ǽ�����Ĺؼ���ע���������Ϣ���н��

 ��У����ϵ�д�
��У����ϵ�д�| A�� | CH4 | B�� | C2H6 | C�� | C2H4 | D�� | C3H6 |
| A�� | �Ҵ������ᣩ ����ʯ�ң����� | |
| B�� | �ұ������ӣ� ��Ũ��ˮ������ | |
| C�� | �����飨�Ҵ��� ������ˮ����Һ | |
| D�� | �����������Ҵ��� �����ᣬŨ���ᣬ���� |
| A�� | ��������ˮ��������������ǣ�ֱ����ˮ��Һ�м�������Cu��OH��2����Һ | |
| B�� | ����֯��ɷ�����˿��������˿�������յķ��� | |
| C�� | �����Ҵ��������������������̼������Һ | |
| D�� | �����������ϩ������������ֱ�ͨ��������Ȼ�̼��Һ�� |
| A�� | ��Ӧ�ġ�H��0 | B�� | �����¶ȣ�ƽ�����淴Ӧ�����ƶ� | ||
| C�� | ��Ӧ�ġ�H��0 | D�� | �����¶ȣ�ƽ��������Ӧ�����ƶ� |
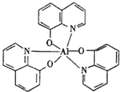 2012��10��1�����ҹ�������̭�׳�ƶ����ø�Ч�����ĵ��·����Ʒ�����·�������в���Mn2+��Cu2+����п���쵥����8-�ǻ�������ȣ�
2012��10��1�����ҹ�������̭�׳�ƶ����ø�Ч�����ĵ��·����Ʒ�����·�������в���Mn2+��Cu2+����п���쵥����8-�ǻ�������ȣ� ���ڷǼ��ԣ�����ԡ����Ǽ��ԡ�������
���ڷǼ��ԣ�����ԡ����Ǽ��ԡ�������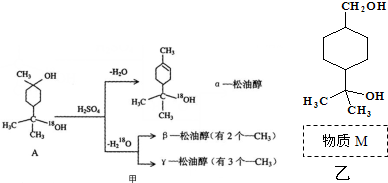
 ��
��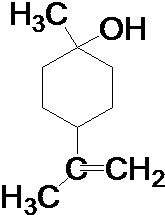 ��
��