��Ŀ����
����������ȷ����
[ ]
A����ˮ���̺���Ԫ����80���֣�����Mg��Br��I�ں�ˮ�е��ܴ����ֱ�ԼΪ1.8��1015t��1��1014t��8��1010t�����ں�ˮ��þ�Ĵ����ܴ�ҵ�ϳ��Ժ�ˮΪԭ����ȡþ����ˣ�þԪ�ر���Ϊ������Ԫ�ء�
B�������ǽ�ԭ��ˮ�����������谷��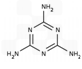 ���׳ơ���������һ�����ǵ��� �ʣ�������������ʳƷ��ҵ�����Ӽ����������谷��������ʳƷ�ӹ�
���׳ơ���������һ�����ǵ��� �ʣ�������������ʳƷ��ҵ�����Ӽ����������谷��������ʳƷ�ӹ�
C�����ڿ��淴ӦN2(g)+3H2(g) 2NH3(g)��������Ũ�ȿ����ӻ���Ӱٷ������Ӷ�ʹ��Ӧ��������
2NH3(g)��������Ũ�ȿ����ӻ���Ӱٷ������Ӷ�ʹ��Ӧ��������
D������������������Ӫ�����ʣ��侧����Ҫ��������ʽ���ڣ���ͬ�İ��������Һ�����������pH������ͬ��������һ���죬����ͨ��������Һ��pH���백����
B�������ǽ�ԭ��ˮ�����������谷��
 ���׳ơ���������һ�����ǵ��� �ʣ�������������ʳƷ��ҵ�����Ӽ����������谷��������ʳƷ�ӹ�
���׳ơ���������һ�����ǵ��� �ʣ�������������ʳƷ��ҵ�����Ӽ����������谷��������ʳƷ�ӹ� C�����ڿ��淴ӦN2(g)+3H2(g)
 2NH3(g)��������Ũ�ȿ����ӻ���Ӱٷ������Ӷ�ʹ��Ӧ��������
2NH3(g)��������Ũ�ȿ����ӻ���Ӱٷ������Ӷ�ʹ��Ӧ�������� D������������������Ӫ�����ʣ��侧����Ҫ��������ʽ���ڣ���ͬ�İ��������Һ�����������pH������ͬ��������һ���죬����ͨ��������Һ��pH���백����
D

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ