��Ŀ����
ij�����ӻ�������ɵĻ����ֻ���ܺ������������е������֣�K����NH4+��Mg2+��Ba2+��Cl����CO32����SO42����Ϊȷ������ɣ�ȷ��ȡ14.82g���������ˮ��300 mL������Һ���ֳ����ȷݷֱ��������ʵ�飺
��1����һ�ݼ���AgNO3��Һ�г�������
��2���ڶ��ݼ�����NaOH��Һ�����ȣ�����ʯ�Ҹ�����ռ������������1.12L
��3�������ݼ�����BaCl2��Һ��������ϴ�ӡ���������Ϊ6.27g����������м��������ᣬ�����ˡ�ϴ�ӡ���������Ϊ2.33 g
��������ʵ�飬�����Ʋⲻ��ȷ����
| A����Һ��c(SO42��)Ϊ0.1mol/L��c(CO32��) Ϊ0.2mol/L |
| B���û�����в���Ba2+��Mg2+ |
| C��һ������NH4+�� K������ȷ��Cl���Ƿ���� |
| D��ʵ�飨3�������м��������ֻ���ˡ���ϴ�ӣ���Գ�NH4+����������Ӻ����IJⶨ���Ӱ�� |
A
�����������������AgNO3��Һ�г���������������Cl-��CO32-��SO42-���ڼ�����NaOH��Һ���Ȳ������壬�����ǰ�������һ���������0.04mol���۲����������2.33gΪ���ᱵ�����ʵ�����0.01mol��6.27g���������ᱵ��̼�ᱵ��̼�ᱵ����Ϊ6.27g-2.33g=3.94g�����ʵ���Ϊ0.02mol����һ������CO32-��SO42-�����һ��û�� Mg2+��Ba2+��c(CO32-)=0.02��0.1=0.02��mol/L�����ٸ��ݵ���غ㣬�����Ϊ��n��+��=n��NH4+��=0.04mol��c��-��=2c(CO32-)+2x(SO42-)=0.06mol����һ����K+������0.02mol���ۺ����Ͽ��Եó���һ�����ڵ�������NH4+��K+��CO32-��SO42-��һ��û�е�����Mg2+��Ba2+�����ܴ���Cl-���ʱ���ѡ��Aѡ�
���㣺���Ӽ��鼰��ؼ��㡣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���ij���塢����dz��ɫ��Һ�У����ܺ������а������ӣ�H����NH4+��Fe3����Ba2����Al3����SO42-��CO32-��I�����ڼ��鷽�����ʱ������������Һ�����ɺ�����(������OH��)�� �� ��
| A��4�� | B��5�� | C��6�� | D��7�� |
���и�������������Һ�д����������
A��Ag����K����NO ��Cl�� ��Cl�� | B��Mg2����Na����Cl����SO42�� |
| C��Cu2����Mg2����OH����Cl�� | D��H����Na����CO32����SO42�� |
�ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʡ����������������±���ʾ��
| ������ | NH�� 4��Na����Mg2�� |
| ������ | OH����NO�� 3��Cl�� |
A���٢� B���ۢ� C���ۢ� D���٢�
�������ӷ���ʽ����ȷ����
| A����һС�������Ʒ���ˮ�У�2Na+2H2O=2Na++2OH-+H2�� |
| B��������ͨ������������Һ�У�Cl2+2OH-=C1-+C1O-+H2O |
| C����CuSO4��Һ�м�������Ba(OH)2��Һ��Ba2++SO42-=BaSO4�� |
| D����ϡ�����м���ͭƬ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O |
ij��������Һ��ֻ���ܺ��У���A13+����Mg2+����Fe3+����Cu2+����H+���� ����
���� �еļ������ӣ������Һ����μ���NaOH��Һ�����������ɳ�����������ͼ��ʾ�������Һ��һ�����е�������
�еļ������ӣ������Һ����μ���NaOH��Һ�����������ɳ�����������ͼ��ʾ�������Һ��һ�����е�������
| A���ڢݢ� | B���ڢݢ� | C���ۢݢ� | D���٢ܢ� |
���з�Ӧ���̷�����ͼ��ʾ��ϵ����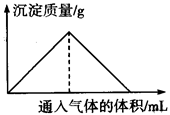
| A����Ba��NO3��2��Һ��ͨ��SO2���������� |
| B����Na2SiO3��Һ��ͨ��HCl���������� |
| C�������ʯ��ˮ��ͨ��CO2���������� |
| D����NaAlO2��Һ��ͨ��HCl���������� |
����H++OH��=H2O���ӷ���ʽ��ʾ�Ļ�ѧ��Ӧ��
| A����������������ķ�Ӧ | B�������������ͭ�ķ�Ӧ |
| C��������������صķ�Ӧ | D�����ᣨ�ѵ��룩���������Ʒ�Ӧ |
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����
| A��ͭ����FeCl3��Һ��Cu+Fe3+=Cu2++Fe2+ |
| B��NaAlO2��Һ��ͨ�����CO2��2AlO2��+CO2+3H2O=2Al(OH)3��+CO32�� |
| C������������Һ�е���Ũ�������������ClO��+Cl��+2H+=Cl2��+H2O |
| D��̼�������Һ�м������ʯ��ˮ��Ca2++2HCO3��+2OH��=CaCO3��+CO32��+2H2O |