��Ŀ����
��12�֣�����ͭ��������������Ӧ�ù㷺��ij�������ú��������ķ�ͭ��Ϊԭ�������������������£�
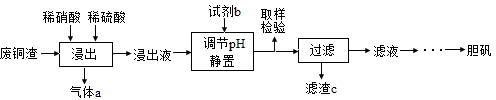
��1��д������ʱͭ��ϡ���ᡢϡ���ᷴӦ��������ͭ�Ļ�ѧ����ʽ�� ��
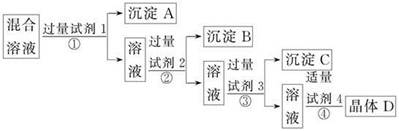
��2��ȡ��������Ϊ��ȷ��Fe3+�Ƿ��������ļ��鷽���� ��
��3������c�� ��
��4������a���Ա�ѭ�����ã��û�ѧ����ʽ��ʾ����a��ѭ�����õ�ԭ��Ϊ��
2NO+O2 =2NO2�� ��
��5��һ���¶��£�����ͭ���ȷֽ�����CuO��SO2���塢SO3�����O2���壬д������ͭ���ȷֽ�Ļ�ѧ����ʽ�� ��
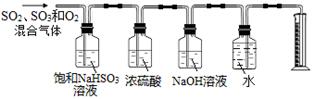
ijͬѧ���������ͼ��ʾ��ʵ��װ�÷ֱ�ⶨ���ɵ�SO2���塢SO3�����������O2����������������в�����֮������˵�����ɣ� ��

��1��д������ʱͭ��ϡ���ᡢϡ���ᷴӦ��������ͭ�Ļ�ѧ����ʽ�� ��
��2��ȡ��������Ϊ��ȷ��Fe3+�Ƿ��������ļ��鷽���� ��
��3������c�� ��
��4������a���Ա�ѭ�����ã��û�ѧ����ʽ��ʾ����a��ѭ�����õ�ԭ��Ϊ��
2NO+O2 =2NO2�� ��
��5��һ���¶��£�����ͭ���ȷֽ�����CuO��SO2���塢SO3�����O2���壬д������ͭ���ȷֽ�Ļ�ѧ����ʽ�� ��
ijͬѧ���������ͼ��ʾ��ʵ��װ�÷ֱ�ⶨ���ɵ�SO2���塢SO3�����������O2����������������в�����֮������˵�����ɣ� ��

��12�֡�
��1��3Cu + 2HNO3 + 3H2SO4 =3CuSO4 + 2NO��+ 4H2O��2�֣�
��2���������еμ�KSCN��Һ������Һ�Ժ�ɫ����Fe3+δ����������Fe3+������2�֣�
��3��Fe(OH)3��2�֣�
��4��3NO2+H2O=2HNO3+NO��2�֣�
��5��3CuSO4 3CuO + SO3��+ 2SO2��+ O2���������𰸾��ɣ���2�֣�
3CuO + SO3��+ 2SO2��+ O2���������𰸾��ɣ���2�֣�
NaHSO3��������SO3��ͻ����ղ���O2�������𰸾��ɣ���2�֣�
��1��3Cu + 2HNO3 + 3H2SO4 =3CuSO4 + 2NO��+ 4H2O��2�֣�
��2���������еμ�KSCN��Һ������Һ�Ժ�ɫ����Fe3+δ����������Fe3+������2�֣�
��3��Fe(OH)3��2�֣�
��4��3NO2+H2O=2HNO3+NO��2�֣�
��5��3CuSO4
 3CuO + SO3��+ 2SO2��+ O2���������𰸾��ɣ���2�֣�
3CuO + SO3��+ 2SO2��+ O2���������𰸾��ɣ���2�֣�NaHSO3��������SO3��ͻ����ղ���O2�������𰸾��ɣ���2�֣�
�����������1������������ԭ��Ӧ���ۣ�ͭ��ϡ���ᡢϡ����Ļ��Һ��Ӧ��������ͭ��һ��������ˮ����ѧ����ʽΪ3Cu + 2HNO3 + 3H2SO4 =3CuSO4 + 2NO��+ 4H2O��
��2��Fe3+�ļ��鷽���ǣ��������еμ�KSCN��Һ������Һ�Ժ�ɫ����Fe3+δ����������Fe3+������
��3����ͭ���к��������������Ե���pHĿ����ʹ�����ӳ�����������c��Fe(OH)3��
��4������a��NO��NO��������Ӧ���ɶ���������������������ˮ�ֵ������NO����ѧ����ʽΪ3NO2+H2O=2HNO3+NO��
��5������ͭ���ȷֽ�����CuO��SO2���塢SO3�����O2���壬��ƽ����ʽ��3CuSO4
 3CuO + SO3��+ 2SO2��+ O2��������NaHSO3��Һ�����ն���������������������Ӧ�����ɶ��������¶���������������
3CuO + SO3��+ 2SO2��+ O2��������NaHSO3��Һ�����ն���������������������Ӧ�����ɶ��������¶���������������
��ϰ��ϵ�д�
�����Ŀ