��Ŀ����
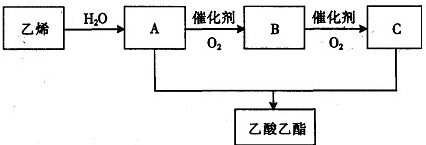
��1��ij��Һ���ԼΪ6mL��Ӧѡ��______mL��Ͳ������Һ������������Ӷ����������6.2mL����ʵ�����Ӧ______6.2mL�����������=��������������
��2������50g25%��NaCl��ҺӦѡ�õ���������Ʒ�У�������ƽ�������룩��ҩ�ס�����ֽ��������______��
��3������500mL2mol?L-1��NaOH��Һ��
��Ӧ��������ƽ��______�п��ٳ�ȡNaOH______g��
�����ƹ������õ��IJ����������ձ�����Ͳ�������⣬����Ҫ______��
����������ᵼ������NaOH��ҺŨ�ȴ���0.2mol?L-1����______��
A��������մ������B��û����ȴ�����¾Ͷ���
C������ʱ���ӿ̶���D��������Һ����ڿ̶���
E������ƿϴ�������������ˮF������ʱ����ˮ�Ӷ����ý�ͷ�ι�������
��2������50g25%��NaCl��ҺӦѡ�õ���������Ʒ�У�������ƽ�������룩��ҩ�ס�����ֽ��������______��
��3������500mL2mol?L-1��NaOH��Һ��
��Ӧ��������ƽ��______�п��ٳ�ȡNaOH______g��
�����ƹ������õ��IJ����������ձ�����Ͳ�������⣬����Ҫ______��
����������ᵼ������NaOH��ҺŨ�ȴ���0.2mol?L-1����______��
A��������մ������B��û����ȴ�����¾Ͷ���
C������ʱ���ӿ̶���D��������Һ����ڿ̶���
E������ƿϴ�������������ˮF������ʱ����ˮ�Ӷ����ý�ͷ�ι�������
��1����ͲѡȡҪѡ������ȡ���������ӽ��ģ�������ȡ6mLˮ��Ӧѡ��10mL��Ͳ����Ͳ�Ķ�����������Һ�尼Һ�����ʹ�����ˮƽ����������Ͳ�����������Ķ���ƫ����ȡ��ʵ��Һ�����ƫС���ʴ�Ϊ��10������
��2�������Ȼ�����ʹ��������ƽ���г�ȡ���ܼ�ˮ��Ҫʹ����Ͳ��ȡ���ܽ������ձ����ò��������н����Լӿ��ܽ⣬�ʴ�Ϊ����Ͳ���ձ���
��3�������������и�ʴ�ԣ��׳��⣬Ӧ��С�ձ��г�����m=CVM=2mol/L��0.5L��40g/mol=40g���ʴ�Ϊ��С�ձ���40��
��ʵ������IJ��裺���㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�������ʵ������Ҫ����ƽ��������ҩ��ȡҩƷ���ձ��ܽ�ҩƷ����Ҫ�������������������Ҫ500mL����ƿ������Һ����Ҫ��ͷ�ιܶ��ݣ��ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��A��������մ�����ۣ���ȡ��������������ƫ��Ũ��ƫ��A��ȷ��
B��û����ȴ�����¾Ͷ��ݣ���Һ���ƫС��Ũ��ƫ��B��ȷ��
C������ʱ���ӿ̶��ߣ���Һ���ƫС��Ũ��ƫ��C��ȷ��
D��������Һ����ڿ̶�����Ӱ�죬ԭ����Һ��ճ�������ϣ���D����
E������ƿ��������ˮϴ����û�к�ɣ���Ӱ�죬Ũ�Ȳ��䣬��E����
F������ʱ����С�ļ�ˮʹҺ����ڿ��ߺ�����������ˮʹ��Һ����̶������У����ʵ��������٣�Ũ��ƫС����F����
�ʴ�Ϊ��AB��
��2�������Ȼ�����ʹ��������ƽ���г�ȡ���ܼ�ˮ��Ҫʹ����Ͳ��ȡ���ܽ������ձ����ò��������н����Լӿ��ܽ⣬�ʴ�Ϊ����Ͳ���ձ���
��3�������������и�ʴ�ԣ��׳��⣬Ӧ��С�ձ��г�����m=CVM=2mol/L��0.5L��40g/mol=40g���ʴ�Ϊ��С�ձ���40��
��ʵ������IJ��裺���㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�������ʵ������Ҫ����ƽ��������ҩ��ȡҩƷ���ձ��ܽ�ҩƷ����Ҫ�������������������Ҫ500mL����ƿ������Һ����Ҫ��ͷ�ιܶ��ݣ��ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
��A��������մ�����ۣ���ȡ��������������ƫ��Ũ��ƫ��A��ȷ��
B��û����ȴ�����¾Ͷ��ݣ���Һ���ƫС��Ũ��ƫ��B��ȷ��
C������ʱ���ӿ̶��ߣ���Һ���ƫС��Ũ��ƫ��C��ȷ��
D��������Һ����ڿ̶�����Ӱ�죬ԭ����Һ��ճ�������ϣ���D����
E������ƿ��������ˮϴ����û�к�ɣ���Ӱ�죬Ũ�Ȳ��䣬��E����
F������ʱ����С�ļ�ˮʹҺ����ڿ��ߺ�����������ˮʹ��Һ����̶������У����ʵ��������٣�Ũ��ƫС����F����
�ʴ�Ϊ��AB��

��ϰ��ϵ�д�
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
�����Ŀ