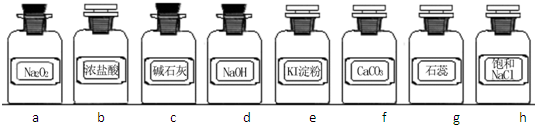
��Ŀ����
��12�֣�ʵ���ҳ���ǿ����������KMnO4��KClO3��MnO2�ȣ�����Ũ����ķ������Ʊ�������ij�о���ѧϰС����̽����Na2O2��Ũ�����Ʊ���������������ѡ�õ�ʵ���Լ���װ�����£����ֵ��ܡ�����ˮ�ԣ���

��1��д����Na2O2��Ũ�����Ʊ������Ļ�ѧ����ʽ_________________________��
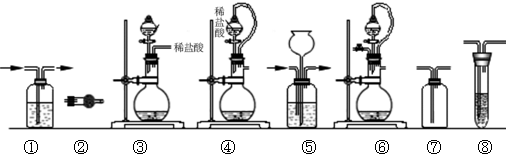
��2������װ��������������__________ ������ţ��迼��ʵ���������װ��ʱ�����к�����Ĵ�������
| ��� | �Ʊ�װ�� | ����װ�� | ����װ��/�Լ� | β������װ�� |
| A | �� | �� | ��/e | �� |
| B | �ۡ��� | �� | ��/g | �� |
| C | �� | �� | ��/e | �� |
| D | �ܡ��� | �� | ��/g | �� |
��4��ijС���Ա������˫��ˮ����������ƽ���ʵ����ã���������������ʵ����ɣ�
�� ___________________________________���� ___________________________________��
��5�����Ƿ�ͬ�⽫Na2O2��Ũ����ķ�Ӧ��Ϊʵ�����Ʊ������ķ���֮һ��___________����ǡ�����������__________________________________________ ��
��12�֣�
��1��Na2O2+4HCl��2NaCl+Cl2��+2H2O��2�֣�
��2��D��4�֣���ѡB����2�֣�
��3��2Na2O2+2H2O=4NaOH+O2������4Na2O2+4HCl��4NaCl +2H2O+ O2������2�֣���
��4���� Na2O2����ˮ��Ӧ����ʹԭ�ϵ������ʽ��� ��1�֣���
��˫��ˮ��Na2O2�����ã�1�֣���
�۲���������Cl2����˫��ˮ���ĵ���������١�
�� Na2O2����ˮ��Ӧ�����ɵ�NaOH�������ᷴӦ��
�� Na2O2����ˮ��Ӧ�����ɵ�NaOH������Cl2
�����������㼴�ɣ��������𰸾��ɣ�ÿ��1�֣�
��5����1�֣��� ���ﲻ�������Է��루1�֣���
����

��1��д����Na2O2��Ũ���ᷴӦ�Ʊ������Ļ�ѧ����ʽ��
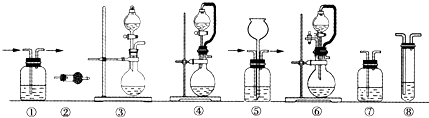
��2������װ��������������
| ��� | �Ʊ�װ�� | ����װ�� | ����װ��/�Լ� | β������װ�� |
| A | �� | �� | ��/e | �� |
| B | �� | �� | ��/e | �� |
| C | �� | �� | ��/e | �� |
| D | �� | �� | ��/e | �� |
��4��ijС���Ա������˫��ˮ����������ƽ���ʵ����ã���������������ʵ����ɣ���
��5�����Ƿ�ͬ�⽫Na2O2��Ũ����ķ�Ӧ��Ϊʵ�����Ʊ������ķ���֮һ��
a��Na2O2 b��Ũ���� c����ʯ�� d��NaOH��Һ e������-KI��Һ
f��CaCO3g��ʯ����Һ h������NaCl��Һ
��1��д��ʵ�����ö������̺�Ũ���ᷴӦ�Ʊ����������ӷ���ʽ
��2��д����Na2O2��Ũ�����Ʊ������Ļ�ѧ����ʽ
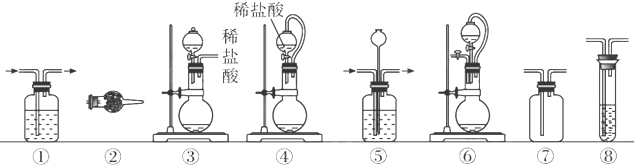
��3������װ��������������
| ��� | �Ʊ�װ�� | ����װ�� | ����װ��/�Լ� | β������װ�� |
| A | �� | �� | ��/e | �� |
| B | �ۢ� | �� | ��/g | �� |
| C | �� | �� | ��/e | �� |
| D | �� | �� | ��/g | �� |
��5��ijС���Ա������˫��ˮ����������ƽ���ʵ����ã���������������ʵ����ɣ�
��
��
��12�֣�ʵ���ҳ���ǿ����������KMnO4��KClO3��MnO2�ȣ�����Ũ����ķ������Ʊ�������ij�о���ѧϰС����̽����Na2O2��Ũ�����Ʊ���������������ѡ�õ�ʵ���Լ���װ�����£����ֵ��ܡ�����ˮ�ԣ���
��1��д����Na2O2��Ũ�����Ʊ������Ļ�ѧ����ʽ_________________________��
��2������װ��������������__________ ������ţ��迼��ʵ���������װ��ʱ�����к�����Ĵ�������
| ��� | �Ʊ�װ�� | ����װ�� | ����װ��/�Լ� | β������װ�� |
| A | �� | �� | ��/e | �� |
| B | �ۡ��� | �� | ��/g | �� |
| C | �� | �� | ��/e | �� |
| D | �ܡ��� | �� | ��/g | �� |
��3��β�������������н϶������ų�������Ҫԭ����û�ѧ����ʽ��ʾΪ��____________ ��
��4��ijС���Ա������˫��ˮ����������ƽ���ʵ����ã���������������ʵ����ɣ�
�� ___________________________________���� ___________________________________��
��5�����Ƿ�ͬ�⽫Na2O2��Ũ����ķ�Ӧ��Ϊʵ�����Ʊ������ķ���֮һ��___________����ǡ�����������__________________________________________ ��