��Ŀ����
A��B��C�Ǵ����Ϥ�����������˶�������ص����ֻ������������Ԫ�ز��������֣���������ת����ϵ��
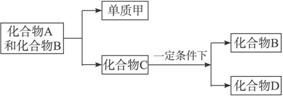
����DҲ���ճ������г������л��������һ�������¿��뵥�ʼ�һ���������±仯��
D+3��![]() 3A+2B
3A+2B
������������⣺
(1)��A��B��C��D���ֻ������У�����Ԫ�������ͬ����_____________(д��������)��
(2)������A��B��ͨ��ʲô;��ת��ΪC�ģ�_____________��д����Ӧ�Ļ�ѧ����ʽ��____________________________________��
(3)Ŀǰ������B����Ȼ���еĺ������������ƣ��Ի��������˲���Ӱ�졣�������ֱ仯����Ҫԭ����___________________________________________________________________��
��1���Ҵ���������
��2��������� 6CO2+6H2O![]() C6H12O6+6O2
C6H12O6+6O2
��3��ɭ�ֱ��ҿ��ķ����ƻ���ֲ��Ĺ�����ã�ͬʱ����ȼ�տ���ȼ�ϣ��Ӷ����¶�����̼���������ߣ����������ЧӦ
����:
�ƶ���Ҫץͻ�ƿڡ������ͻ�ƿ�һ������(3)����ɣ�������֪ʶ����֪BΪCO2������A��B��C�������˶�������أ�A��C����ΪH2O�ȡ����A��B��ת��ΪC����ȷ��AΪH2O��CΪC6H12O6����֮���Ƴ�DΪ�Ҵ�����ΪO2��

 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�


