��Ŀ����
��֪��Ӧ�٣�CO(g)��CuO(s)CO2(g)��Cu(s)�ͷ�Ӧ�ڣ�H2(g)��CuO(s)Cu(s)��H2O(g)����ͬ��ij�¶��µ�ƽ�ⳣ���ֱ�ΪK1��K2�����¶��·�Ӧ�ۣ�CO(g)��H2O(g)CO2(g)��H2(g)��ƽ�ⳣ��ΪK��������˵����ȷ����(����)
A����Ӧ�ٵ�ƽ�ⳣ��K1��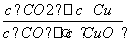
B����Ӧ�۵�ƽ�ⳣ��K��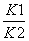
C�����ڷ�Ӧ�ۣ�����ʱ���¶����ߣ�H2Ũ�ȼ�С����÷�Ӧ���ʱ�Ϊ��ֵ
D�����ڷ�Ӧ�ۣ����º����£�����ѹǿ��H2Ũ��һ����С
������ѡB������дƽ�ⳣ������ʽʱ�������岻�ܱ�ʾ��ƽ�ⳣ������ʽ�У�A�������ڷ�Ӧ�ۣ���Ӧ�٣���Ӧ�ڣ����ƽ�ⳣ��K��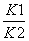 ��B��ȷ����Ӧ���У��¶����ߣ�H2Ũ�ȼ�С����ƽ�������ƶ������淴ӦΪ���ȷ�Ӧ������ӦΪ���ȷ�Ӧ��ӦΪ��H<0��C�����ڷ�Ӧ�ۣ��ں��º����£�����ѹǿ�������ϡ�����壬��ƽ�ⲻ�ƶ���H2��Ũ�Ȳ��䣬D����
��B��ȷ����Ӧ���У��¶����ߣ�H2Ũ�ȼ�С����ƽ�������ƶ������淴ӦΪ���ȷ�Ӧ������ӦΪ���ȷ�Ӧ��ӦΪ��H<0��C�����ڷ�Ӧ�ۣ��ں��º����£�����ѹǿ�������ϡ�����壬��ƽ�ⲻ�ƶ���H2��Ũ�Ȳ��䣬D����

Ϊ�ᴿ�������ʣ������ڵ����������ʣ�����ѡ�õij����Լ��ͷ��뷽������ȷ���ǣ� ��
| ���ᴿ������ | �����Լ� | ���뷽�� | |
| A | �廯����Һ��NaI�� | ��ˮ��CCl4 | ��ȡ����Һ |
| B | �Ȼ����Һ��FeCl3�� | ����������Һ | ���� |
| C | ����������NO�� | ���� | ͨ�����O2 |
| D | ̼��������Һ��̼���ƣ� | ������̼ | ����Һ��ͨ�����CO2 |
 ��D(g)����2 min��B��Ũ�ȼ���0.6 mol/L���Դ˷�Ӧ���ʵı�ʾ��ȷ����(����)
��D(g)����2 min��B��Ũ�ȼ���0.6 mol/L���Դ˷�Ӧ���ʵı�ʾ��ȷ����(����) 2CO2(g)��N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯������ͼ��ʾ��
2CO2(g)��N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯������ͼ��ʾ��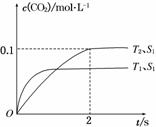
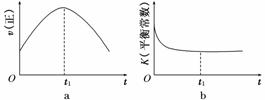
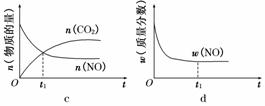
 ���������������������CH4����ԭNOx�������������������Ⱦ��
���������������������CH4����ԭNOx�������������������Ⱦ�� .9 kJ/mol
.9 kJ/mol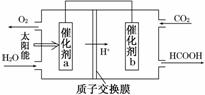
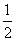 lg(
lg(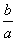 ) B.
) B.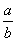 )
) NH3��H2O��������ʣ�
NH3��H2O��������ʣ�