��Ŀ����
����Ŀ��ijͬѧ����ͼ��ʾװ�ü��������������(FeC2O42H2O������ɫ)���ȷֽ�IJ��ֲ������˵����ȷ�� ��( )
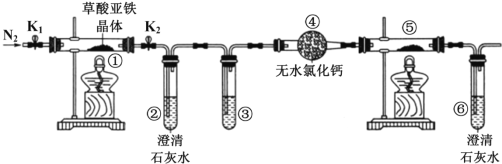
A. ͨ��N2 ����ҪĿ���Ƿ�ֹ�����е�ˮ�����Բ���������Ӱ��
B. �������е���ˮ CaC12 ������ˮ����ͭ�ɼ���ֽ����ɵ�ˮ����
C. �������� CO ���ɣ��ۺ͢��зֱ�ʢ������NaOH ��Һ��CuO
D. ʵ��������е���ɫ��ĩ��ȫ��ɺ�ɫ�������һ��Ϊ��
���𰸡�C
��������
�����е�������������̼�Բ��������Ӱ�죻װ���ڢ�������ˮ����������ˮ�����ļ���������ʢ������NaOH��Һ�����Խ�������̼��ȥ��������CuO��죬����ʯ��ˮ����ǣ�����CO�����������������������������Ǻ�ɫ���壻
ͨ��N2����ҪĿ���Ƿ�ֹ�����е�������������̼�Բ�����������Ӱ������A����װ���ڢ�������ˮ����������ˮ�����ļ��飬���Խ����е���ˮCaC12 ������ˮ����ͭҲ���ܼ���ֽ����ɵ�ˮ��������B��������ʢ������NaOH��Һ�����Խ�������̼��ȥ��������CuO��죬����ʯ��ˮ����ǣ���ɼ���CO����C��ȷ�����������������������������Ǻ�ɫ���壬���е���ɫ��ĩ��ȫ��ɺ�ɫ�����ﲻһ��Ϊ������D����

 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�����Ŀ�����ܵĴ�С�����ڼ��㻯ѧ��Ӧ�ķ�Ӧ�ȣ���H����
��ѧ�� | Cl��Cl | H��H | H��Cl | N��N |
����/kJ��mol-1 | 243 | 436 | 431 | 946 |
��1�������ϱ��е������жϷ�����ӦH2(g) + Cl2(g)�� 2HCl(g)�����У�������1molHCl����ʱ��Ҫ___�����������������ų��� ��____kJ������
��2����֪N2 (g)+3H2(g) = 2NH3(g) ��H=-92 kJ/mol����N��H���ļ�����___kJ��mol-1��
��3��1molNO2�����1mol CO���巴Ӧ����CO2�����NO��������������仯����ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��____��

��4����֪�ڳ��³�ѹ�£�
��2CH3OH(l)��3O2(g)��2CO2(g)��4H2O(g) ��H1
��2CO(g)+O2(g)��2CO2(g) ��H2
��H2O(g)��H2O(l) ��H3
��CH3OH(l)��O2(g)= CO(g) + 2H2O(l) ��H=_______���ú���H1����H2����H3��ʽ�ӱ�ʾ����