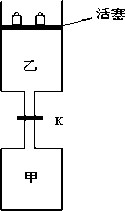
��Ŀ����
����ͼ���رշ���Kʱ������г���1.5 mol A��3.5 mol B,�����г���3 mol A��7 mol B,��ʼʱ�����������������ΪV L������ͬ�¶Ⱥ��д������ڵ������£��������и��Է������з�Ӧ��3A��g��+2B��g��
��1������B��ת����Ϊ______________________________��
��2������D������C�����ʵ����ıȽϣ�______________________________�����ȡ���ǰ�ߴ����ߴ�����������______________________________��
��3����K����һ��ʱ�����´ﵽƽ�⣨��ʱ���ҵ����Ϊ���ú�V�Ĵ���ʽ��ʾ����ͨ���е�����������Բ��ƣ�______________________________________________________��
��1��20%
��2�����ߴ� ��ʼʱ��������ѹǿ�Ǽ�������2������Ӧ��ƽ���������ѹǿ�Ǽ�����ѹǿ��2�����࣬�������ʵ�ת���ʽϼ״�ö�
��3��0.29V
������
��1��3A��g��+2B��g��![]() C��g��+2D��g�� ��V
C��g��+2D��g�� ��V
3 2 1 2 2
�� 3 mol 7 mol 0 ?0
ת 2.1 mol? 1.4 mol?0.7 mol?1.4 mol��3+7����(1-0.86) mol=1.4 mol
����B��ת����![]() ��100%=20%
��100%=20%
(3)����������Ϊһ�壬��ԭ�������ǵ�Чƽ�⣬��
![]() ��V(��)=
��V(��)=![]() ��V(��)=
��V(��)=![]() ��0.86 V=1.29 V
��0.86 V=1.29 V
ƽ����ҵ����V(��)=V(��)-V(��)=1.29 V-V=0.29 V��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�