��Ŀ����
��һ�½̲�����һ����ʾʵ�飺����֬�ް�סԼ0��2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ��������
��1����ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�a�����������ɣ�b��________��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ�ǣ�________��
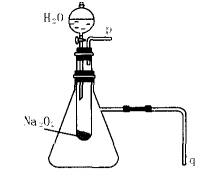
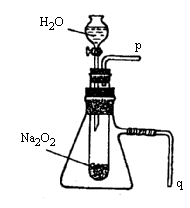
��2��ij�о���ѧϰС������ͼ10װ�ý���ʵ�飬��֤���������ۡ�
��������֤����a��ʵ�鷽���ǣ�________��
��������֤����b��ʵ�鷽���������ǣ�________
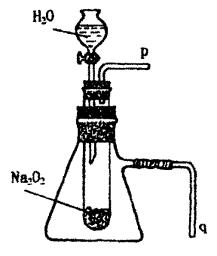
��3��ʵ�飨2�����Թ��м�ˮ��������ȫ�ܽ��Ҳ������������ɺ�ȡ���Թܣ����Թ��е����̪��Һ��������Һ��죬��ɫ���ʡ�Ϊ̽��������С��ͬѧ�Ӳ����й������е�֪��Na2O2��H2O��Ӧ������H2O2��H2O2����ǿ�����Ժ�Ư���ԡ������һ����ʵ�飬֤��Na2O2������H2O��ַ�Ӧ�����Һ����H2O2���ڣ�ֻҪ���г�ʵ�����õ��Լ����۲쵽������
�Լ���________������________��
������
��1���÷�Ӧ�Ƿ��ȷ�Ӧ 2Na2O2��2H2O�T�T4NaOH��O2�� ��2���ٽ����л��ǵ�ľ�����������ܿ�p����ľ����ȼ �ڽ�������q����ˮ�У���Ӧ�����йܿڴ�������ð�� ��3��Na2S��Һ ��Һ����ǣ����ǣ���ɫ������������ɫ�ȣ�
|

��һ�½̲�����һ��ʾʵ�飬����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ��������
��1��������ʵ���������ó����йع������Ƹ�ˮ��Ӧ�Ľ����ǣ�
��һ�����������ɣ��ڶ��� ��Na2O2��ˮ��Ӧ�Ļ�ѧ����ʽ�� ��
���л�ԭ���� ���������� ��
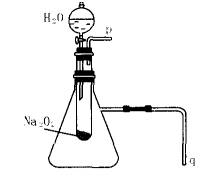
��2��ij�о���ѧϰС������Aͼ��ʾװ�ã����������ã�����ʵ�飬��֤���������ۡ�������֤��һ�����۵�ʵ�鷽���ǣ�
������֤�ڶ������۵�ʵ�鷽���ǣ�
��3��ʵ�飨2�����Թ��м�ˮ��������ȫ�ܽ��Ҳ������������ɺ�ȡ���Թܣ����Թ��е����̪��Һ��������Һ�ȱ�����ɫ��Ϊ̽����ԭ��С��ͬѧ�Ӳ����й������е�֪��Na2O2��ˮ��Ӧ������H2O2��H2O2����ǿ�����Ժ�Ư���ԡ������һ����ʵ�飬��֤Na2O2������ˮ��ַ�Ӧ�����Һ����H2O2���ڡ���ֻҪ��д��ʵ�����õ��Լ����۲쵽������
�Լ��� ��
���� ��
��4����С��ͬѧ����ö����ķ���̽��Na2O2��ˮ��Ӧ�����Һ�з���H2O2����ʵ�鷽��Ϊ����ȡ2.6g Na2O2���壬ʹ֮��������ˮ��Ӧ����������O2�������������ֵ�Ƚϣ����ɵó����ۡ�
|
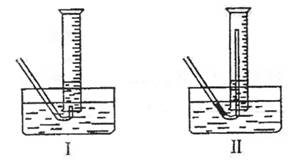
�ٲ����������ʱ��������Թܺ���Ͳ�ڵ����嶼��ȴ������ʱ���У�Ӧѡ����ͼװ���еģ����Ե�������Ͳ����ռ������� ������ţ��������� ��
�����ڱ�״���²�������������Ӧѡ�õ���Ͳ�Ĵ�С���Ϊ
��ѡ�100mL����200mL����500mL����1000mL������