��Ŀ����
�ϵ��¶��£�����ͨ��ʯ�����п��Ƶ�Ư�ۣ��÷�ӦΪ���ȷ�Ӧ��ijУ�ס�������ѧС�����200 mL 12 mol/L������17.4 g MnO2�ڼ��������·�Ӧ�Ʊ������������Ʊ��������������ʯ���鷴Ӧ��ȡƯ�ۣ���ϡNaOH��Һ���ղ��������������ʵ�������֣��ټס��������Ƶõ�Ư����Ca(ClO)2����������С������ֵ���ڼ����ڽϸ��¶��½������������ʯ���鷴Ӧ�����ƵõIJ�Ʒ��Ca(ClO3)2�ĺ����ϸߡ��Իش��������⣺��1������ʵ���������������Ƶ�Ca(ClO)2���ٿ�?
��2��ʵ�������õ���Ca(ClO)2����������С������ֵ���Լ�Ҫ���������ԭ��
��2��ʵ�������õ���Ca(ClO)2����������С������ֵ���Լ�Ҫ���������ԭ��
��1�� n(HCl) =" 12��0.2" =" 2.4" mol��n(MnO2) =" 17.4=" 0.2 mol
MnO2 + 4 HCl(Ũ) �� MnCl2 + Cl2��+ 2H2O
n(MnO2)��n(HCl) =" 2.4" mol��0.2 mol =" 12��1" �� 4��1 ����ŨHCl������Ӧ����MnO2���㡣�����Ʊ������Ļ�ѧ����ʽ��n(Cl\2) = n(MnO2) =" 0.2" mol
������2Cl2 + 2Ca(OH)2�� Ca(ClO)2 + CaCl2 + 2H2O n[Ca(ClO)2] =" 1/2" n(Cl2) =" 1/2��0.2" mol =" 0.1" mol m[Ca(ClO)2] =" 143��" 0.1 =" 14.3" g
��2�������ŷ�Ӧ���У��¶����ߣ������������Ca(ClO3)2��
��Cl2δ��ʯ������ȫ��Ӧ������Cl2��NaOH��Һ����
MnO2 + 4 HCl(Ũ) �� MnCl2 + Cl2��+ 2H2O
n(MnO2)��n(HCl) =" 2.4" mol��0.2 mol =" 12��1" �� 4��1 ����ŨHCl������Ӧ����MnO2���㡣�����Ʊ������Ļ�ѧ����ʽ��n(Cl\2) = n(MnO2) =" 0.2" mol
������2Cl2 + 2Ca(OH)2�� Ca(ClO)2 + CaCl2 + 2H2O n[Ca(ClO)2] =" 1/2" n(Cl2) =" 1/2��0.2" mol =" 0.1" mol m[Ca(ClO)2] =" 143��" 0.1 =" 14.3" g
��2�������ŷ�Ӧ���У��¶����ߣ������������Ca(ClO3)2��
��Cl2δ��ʯ������ȫ��Ӧ������Cl2��NaOH��Һ����
��

��ϰ��ϵ�д�
�����Ŀ
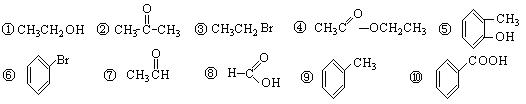
 ����ϵͳ�������������� ��
����ϵͳ�������������� ��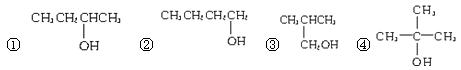
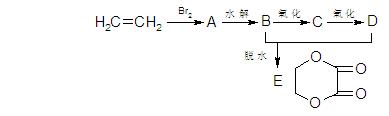
 ������
������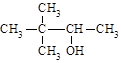 2,2-����-3-����
2,2-����-3-���� 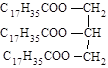 Ӳ֬�����֬
Ӳ֬�����֬

 ��4���üױ���TNT�ķ�Ӧ����ʽ_______________________����Ӧ���ͣ�__________��
��4���üױ���TNT�ķ�Ӧ����ʽ_______________________����Ӧ���ͣ�__________��
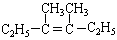 ���������������·�����Ӧ����ģ������д���÷�Ӧ�ķ���ʽ��
���������������·�����Ӧ����ģ������д���÷�Ӧ�ķ���ʽ��