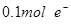��Ŀ����
��16�֣���ͼ��ʾ��ͨ��5 min�缫5������������2.16 g���ش�
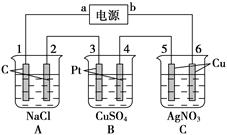
(1)��Դ��a��________����C����________�ء�
A�������缫��ӦʽΪ__________________�������缫��ӦʽΪ__________________��
C�������缫��Ӧʽ__________________�������缫��ӦʽΪ_____________________��
(2)���B���й��ռ���224 mL����(��״��)������Һ���Ϊ200 mL(�����������Һ�������)����ͨ��ǰ��Һ��Cu2�������ʵ���Ũ��Ϊ______________________��
(3)���A����ҺҲ��200 mL(����������Һ�������)����ͨ�����Һ��pHΪ[________��

(1)��Դ��a��________����C����________�ء�
A�������缫��ӦʽΪ__________________�������缫��ӦʽΪ__________________��
C�������缫��Ӧʽ__________________�������缫��ӦʽΪ_____________________��
(2)���B���й��ռ���224 mL����(��״��)������Һ���Ϊ200 mL(�����������Һ�������)����ͨ��ǰ��Һ��Cu2�������ʵ���Ũ��Ϊ______________________��
(3)���A����ҺҲ��200 mL(����������Һ�������)����ͨ�����Һ��pHΪ[________��
(1)������⡡2Cl����2e��===Cl2�� 2H����2e��===H2��
Cu��2e��===Cu2�� ��2Ag����2e��===2Ag (2)0.025 mol��L��1��(3)13
Cu��2e��===Cu2�� ��2Ag����2e��===2Ag (2)0.025 mol��L��1��(3)13
��1���缫5������������2.16 g��˵���缫5����������缫6������������b�ǵ�Դ�ĸ�����a�ǵ�Դ�ĸ�������缫1��3���������缫2��4��������A�������������ӷŵ磬���������������������ӷŵ磬����������C��������ͭ�����ڻ��Ե缫��ͭʧȥ���ӡ���������Һ�е������ӷŵ磬��������
��2��B�ǵ������ͭ��������OH���ŵ������������������ͭ���ӷŵ磬����ͭ��Ȼ���������ӷŵ�����������缫5������������2.16 g����������������0.02mol��ת�Ƶ�����0.02mol����B������������0.005mol�������0.005mol��22.4L/mol��112ml����������Ҳ��112ml�����ݵ����غ��֪��ͭ���ӵõ��ĵ�����0.02mol��0.005mol��2��0.01mol�����ͭ���ӵ����ʵ�����0.005mol��������ͭ��Ũ����0.005mol��0.2L��0.025mol/L��
��3�����ݷ�Ӧʽ2NaCl��2H2O 2NaOH��H2����Cl2����֪�����ɵ�����������0.02mol����Ũ����0.1mol/L������pH��13��
2NaOH��H2����Cl2����֪�����ɵ�����������0.02mol����Ũ����0.1mol/L������pH��13��
��2��B�ǵ������ͭ��������OH���ŵ������������������ͭ���ӷŵ磬����ͭ��Ȼ���������ӷŵ�����������缫5������������2.16 g����������������0.02mol��ת�Ƶ�����0.02mol����B������������0.005mol�������0.005mol��22.4L/mol��112ml����������Ҳ��112ml�����ݵ����غ��֪��ͭ���ӵõ��ĵ�����0.02mol��0.005mol��2��0.01mol�����ͭ���ӵ����ʵ�����0.005mol��������ͭ��Ũ����0.005mol��0.2L��0.025mol/L��
��3�����ݷ�Ӧʽ2NaCl��2H2O
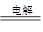 2NaOH��H2����Cl2����֪�����ɵ�����������0.02mol����Ũ����0.1mol/L������pH��13��
2NaOH��H2����Cl2����֪�����ɵ�����������0.02mol����Ũ����0.1mol/L������pH��13��
��ϰ��ϵ�д�
 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
�����Ŀ
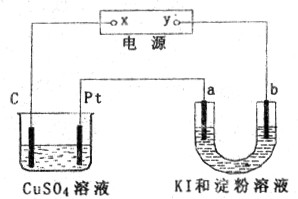
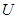 �ι��еı���
�ι��еı��� ��Һ�����з�̪��Һ��������ͼ������������ȷ����
��Һ�����з�̪��Һ��������ͼ������������ȷ����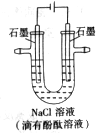
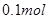 ����ʱ��������·��ת����
����ʱ��������·��ת����