��Ŀ����
��һ���װ����ͼ��ʾ��ͼ��Bװ����ʢ��
��1��A�з�����Ӧ�Ļ�ѧ����ʽΪ________________________________________________��
��2����B�й۲쵽��������_______________________________________________________��
��3�������£����ӵ�ʼ��ʱ��Ϊtʱ��A��Bװ���й��ռ�������
������C�˱���˵��������2I--2e-![]() I2ʹ���۱�������C��Ϊ������D��Ϊ�������ݴ����ȷ����Դ������������A��B����������������BʯīΪ����������2H++2e-
I2ʹ���۱�������C��Ϊ������D��Ϊ�������ݴ����ȷ����Դ������������A��B����������������BʯīΪ����������2H++2e-![]() H2��������OH-����CuΪ�������ʲ����ܽ⡣����A��FeΪ��������Ag������PtΪ��������O2���������ڲ�����O2ΪH2��
H2��������OH-����CuΪ�������ʲ����ܽ⡣����A��FeΪ��������Ag������PtΪ��������O2���������ڲ�����O2ΪH2��![]() ����O2����Ϊ��
����O2����Ϊ��![]() =0.002 5 mol��ͬʱ��ʹA��H+��Ϊ4��0.002 5 mol=0.01 mol������A��c(H+)=0.01 mol��L-1,pH=2��
=0.002 5 mol��ͬʱ��ʹA��H+��Ϊ4��0.002 5 mol=0.01 mol������A��c(H+)=0.01 mol��L-1,pH=2��
�𰸣���1��4AgNO3+2H2O![]() 4Ag��+O2��+4HNO3
4Ag��+O2��+4HNO3
��2��ʯī�缫���������ݲ�����ͭ�缫��Χ��Һ�����ɫ��һ��ʱ���U�ι��²�����ɫ��������
��3��2

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
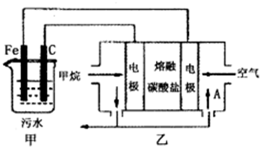 ��2013?��ģ�⣩��ҵ�ϲ��õ�һ����ˮ�����������£�������ˮ��pH��5.0��6.0֮�䣬ͨ���������Fe��OH��3��Fe��OH��3���������ԣ�����������������������о���ˮ�����ã��������������ݰ���ˮ�����������ˮ���γɸ����㣬��ȥ����Ʋ���������㣬�����˸�ѡ���������ã�ij����С���ø�ԭ��������ˮ�����װ����ͼ��ʾ������˵����ȷ���ǣ�������
��2013?��ģ�⣩��ҵ�ϲ��õ�һ����ˮ�����������£�������ˮ��pH��5.0��6.0֮�䣬ͨ���������Fe��OH��3��Fe��OH��3���������ԣ�����������������������о���ˮ�����ã��������������ݰ���ˮ�����������ˮ���γɸ����㣬��ȥ����Ʋ���������㣬�����˸�ѡ���������ã�ij����С���ø�ԭ��������ˮ�����װ����ͼ��ʾ������˵����ȷ���ǣ�������




