��Ŀ����
ȡA��B�������ʵ���Ũ����ȵ�NaOH��Һ�������Ϊ50 mL���ֱ�������ͨ��һ������CO2���ٷֱ�ϡ��Ϊ100 mL��
(1)��NaOH��Һ��ͨ��һ������CO2����Һ�е����ʵ���ɿ����ǣ�
�� ���� ���� ���� ��
(2)��ϡ�ͺ����Һ�зֱ���μ���0.1 mol��L��1�����ᣬ������CO2�����(��״��)����������������ϵ��ͼ��ʾ��
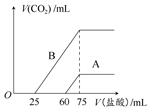
�ٷֱ�����������������Һ�е������� ��ԭNaOH��Һ�����ʵ���Ũ���� ��
��A���߱�����ͨ��CO2����Һ�е������� �������ᷴӦ����CO2���������� mL(��״��)��
��B���߱�����ԭNaOH��Һͨ��CO2���������ʵĻ�ѧʽΪ �������ʵ���֮��Ϊ ��
(1)��NaOH��Һ��ͨ��һ������CO2����Һ�е����ʵ���ɿ����ǣ�
�� ���� ���� ���� ��
(2)��ϡ�ͺ����Һ�зֱ���μ���0.1 mol��L��1�����ᣬ������CO2�����(��״��)����������������ϵ��ͼ��ʾ��

�ٷֱ�����������������Һ�е������� ��ԭNaOH��Һ�����ʵ���Ũ���� ��
��A���߱�����ͨ��CO2����Һ�е������� �������ᷴӦ����CO2���������� mL(��״��)��
��B���߱�����ԭNaOH��Һͨ��CO2���������ʵĻ�ѧʽΪ �������ʵ���֮��Ϊ ��
(1)��Na2CO3��NaOH ��Na2CO3��Na2CO3��NaHCO3��NaHCO3 (2)��NaCl��NaCl��HCl��0.15 mol��L��1��NaOH��Na2CO3��33.6 ��Na2CO3��NaHCO3��1��1��
(1)��NaOH��Һ��ͨ��һ������CO2�����ķ�Ӧ�У�2NaOH��CO2=Na2CO3��H2O��Na2CO3��H2O��CO2=2NaHCO3(CO2����)���÷�Ӧ���̷ֲ����У��ʾݷ�Ӧ��CO2�����IJ�ͬ����Һ�е�������ɿ��ܳ������¼����������Na2CO3��NaOH����Na2CO3����Na2CO3��NaHCO3����NaHCO3��(2)������֪����������ķ�Ӧ��Ϊ��NaHCO3��HCl=NaCl��H2O��CO2������Na��ClԪ���غ�֪��c(NaOH)��n(NaOH)/V(NaOH)��n(HCl)/V(NaOH)��(75��10��3L��0.1 mol��L��1)/(50��10��3L)��0.15 mol��L��1����A����NaHCO3��HCl=NaCl��H2O��CO2��֪�����ɵ�n(CO2)��15��10��3L��0.1 mol��L��1��1.5��10��3 mol(��33.6 mL)����A�иտ�ʼ��������ʱn(NaHCO3)��1.5��10��3 mol������������δ�����������������HCl�������Ϊ1��4����֪A������ΪNaOH��Na2CO3�����B����֪������������δ�����������������HCl�������Ϊ2��1(Na2CO3��HCl=NaHCO3��NaCl��NaHCO3��HCl=NaCl��H2O��CO2��)����B������ΪNa2CO3��NaHCO3����n(Na2CO3)��n(NaHCO3)��1��1��

��ϰ��ϵ�д�
�����Ŀ