��Ŀ����
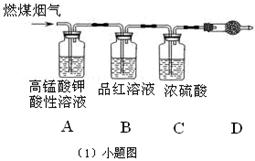
��14�֣�����ͼװ����ʾ����������ȼ�ϵ��B���е�ij���ʵ�飺
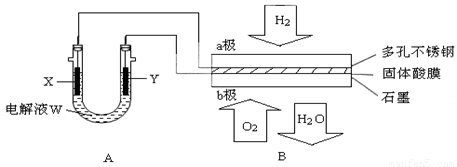
(1)�����Bʹ�����ǰ����(Li2NH)������Ϊ������ϣ��䴢��ԭ���ǣ�Li2NH��H2��LiNH2��LiH��������˵������ȷ����________��
(2)�ڵ��B����ʱ��
�����ù���Ca(HSO4)2Ϊ����ʴ���H�����������________��������H����________���ƶ������a����b����
��b���ϵĵ缫��ӦʽΪ��________________________________
�����·�У�ÿת��0.1 mol���ӣ���a������________�ϵ�H2����״���£���
(3)��A��X��Y���Ƕ��Ե缫�����ҺW�ǵ��з�̪�ı���NaCI��Һ����B����ʱ��
�ٵ�����X���ϵĵ缫��Ӧʽ�� ________________________________��
��X����߹۲쵽�������� _____________________________________��
�ڼ���Y�缫�Ϸ�Ӧ����ķ����� _________________________________��
����A�����������ı䣬ֻ���缫Y������������ʵ�ֵ�ʵ��Ŀ����_____________________��

(1)�����Bʹ�����ǰ����(Li2NH)������Ϊ������ϣ��䴢��ԭ���ǣ�Li2NH��H2��LiNH2��LiH��������˵������ȷ����________��
| A��Li2NH��N�Ļ��ϼ���-1�� | B���÷�Ӧ��H2�������������ǻ�ԭ�� |
| C��Li�� ��H�������Ӱ뾶��� | D���˷������ƿ�����ԭ����ͬ |
�����ù���Ca(HSO4)2Ϊ����ʴ���H�����������________��������H����________���ƶ������a����b����
��b���ϵĵ缫��ӦʽΪ��________________________________
�����·�У�ÿת��0.1 mol���ӣ���a������________�ϵ�H2����״���£���
(3)��A��X��Y���Ƕ��Ե缫�����ҺW�ǵ��з�̪�ı���NaCI��Һ����B����ʱ��
�ٵ�����X���ϵĵ缫��Ӧʽ�� ________________________________��
��X����߹۲쵽�������� _____________________________________��
�ڼ���Y�缫�Ϸ�Ӧ����ķ����� _________________________________��
����A�����������ı䣬ֻ���缫Y������������ʵ�ֵ�ʵ��Ŀ����_____________________��
��1��B ��2����a��b �� O2 + 4H+ + 4e- = 2H2O �� 1.12 ��3���� 2H++2e- = H2������Һ���
������ʪ��KI������ֽ�ӽ�Y��������������֤������Cl2�� �� ��Fe(OH)2
������ʪ��KI������ֽ�ӽ�Y��������������֤������Cl2�� �� ��Fe(OH)2
�����������1��A��Li2NH��N�Ļ��ϼ�Ϊ-3�ۣ���A����B����Ӧ��H2�ֱ�����LiNH2��LiH��LiNH2��HΪ+1�ۣ�LiH��H�Ļ��ϼ�Ϊ-1�ۣ���Ӧ��H2�������������ǻ�ԭ������B��ȷ��C��LiH�е������Ӻ������Ӻ�������Ų���ͬ���˵����Խ�뾶ԽС���������Ӱ뾶С�������Ӱ뾶����C����D���˷�����������������ԭ��Ӧ��H2ת��Ϊ������ϣ�������ѧ�仯������ƿ���⽫��Һ����Ϊ�����仯��ԭ����ͬ����D����ѡB��
��2����ͨ�������ĵ缫Ϊ��صĸ���������������ԭ��Ӧ����ӦΪH2-2e-��2H+��ͨ�������ĵ缫Ϊ��ص�����������������Ӧ����ӦΪO2+4e-+4H+=2H2O����ع���ʱ������ͨ�����·�Ӹ�����������������a������b�����������Һ���������������ƶ�����H+��a��ͨ�����������ʴ��ݵ�b����
��b��Ϊ����������������Ӧ���缫��ӦO2+4e-+4H+=2H2O��
��ת�Ƶ���غ㣬������֪ÿת��2mol��������1mol H2����ÿת��0.1mol���ӣ���a������0.05mol H2����1.12L H2��
��3��������֪��a��Ϊ��Դ������b��Ϊ��Դ�����ٵ�ⱥ��ʳ��ˮ�к͵�Դ�ĸ���a�������ĵ缫X�����������õ缫�������ӷ����õ��ӵĻ�ԭ��Ӧ����2H++2e-=H2������Һ��H+����Ũ�ȼ�С��������ǿ����̪��졣
�ں͵�Դ�����������ĵ缫Y�����������õ缫�������ӷ���ʧ���ӵ�������Ӧ����2Cl--2e-=Cl2�����ɲ���Ϊ���������鷽ʽΪ����ʪ��KI������ֽ�ӽ�Y��������������֤������Cl2����
��Y������ʧȥ���ӵ�������Ӧ���ϼ����ߣ��������������ӣ���Һ���д������������Ӻ��������ӽ��������������������������Ŀ��Ϊ��Fe(OH)2��

��ϰ��ϵ�д�
�����Ŀ




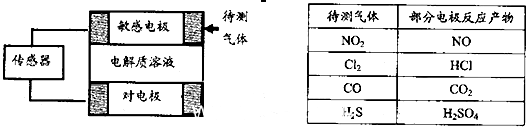
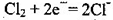
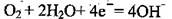
 LaNi5H+NiOOH�������й�˵������ȷ����
LaNi5H+NiOOH�������й�˵������ȷ����