��Ŀ����
��9�֣��������ٻ��š����ֵķз�����ķ���Prius����������϶������������õ綯������ȼ������߽���ƶ����֡��������»����ʱ���綯���ṩ�ƶ��������������͵����ģ���ɲ��������ʱ�綯�����ڳ��״̬��
��1����϶����� ����ȼ��������Ϊȼ�ϣ�����(������C8H18��)��������ַ�Ӧ��ÿ����1 molˮ��������569.1 kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ_______ ��
����ȼ��������Ϊȼ�ϣ�����(������C8H18��)��������ַ�Ӧ��ÿ����1 molˮ��������569.1 kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ_______ ��
��2����϶������ĵ綯��Ŀǰһ��ʹ�õ��������أ������ز������Ļ�����Ϊ�������������(��M��ʾ)Ϊ��������Һ(��ҪΪKOH)Ϊ���Һ�������س�ŵ�ԭ��ʾ����ͼ�����ܷ�Ӧʽ��:
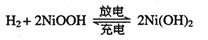
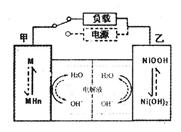
����������Ϣ�жϣ���϶��������»����ʱ���ҵ缫��Χ��Һ��pH��_______(����������䡱��С��)���õ缫�ĵ缫��ӦʽΪ_________________��
��3�����˽�������β���е�CO�����ü���������ȷֽ�ķ�����������֪�ȷֽⷴӦCO(g)��C(s)��O2(g)�ġ�H����110.5kJ/mol����S����0.089kJ��mol��1��K��1����һ�����������Ƿ���У�____________(ѡ������С������С�)������

��1����϶�����
 ����ȼ��������Ϊȼ�ϣ�����(������C8H18��)��������ַ�Ӧ��ÿ����1 molˮ��������569.1 kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ_______ ��
����ȼ��������Ϊȼ�ϣ�����(������C8H18��)��������ַ�Ӧ��ÿ����1 molˮ��������569.1 kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ_______ ����2����϶������ĵ綯��Ŀǰһ��ʹ�õ��������أ������ز������Ļ�����Ϊ�������������(��M��ʾ)Ϊ��������Һ(��ҪΪKOH)Ϊ���Һ�������س�ŵ�ԭ��ʾ����ͼ�����ܷ�Ӧʽ��:
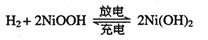

����������Ϣ�жϣ���϶��������»����ʱ���ҵ缫��Χ��Һ��pH��_______(����������䡱��С��)���õ缫�ĵ缫��ӦʽΪ_________________��
��3�����˽�������β���е�CO�����ü���������ȷֽ�ķ�����������֪�ȷֽⷴӦCO(g)��C(s)��O2(g)�ġ�H����110.5kJ/mol����S����0.089kJ��mol��1��K��1����һ�����������Ƿ���У�____________(ѡ������С������С�)������


��1��C8H18(l)��25/2O2(g)��8CO2(g)��9H2O(g) ��H����5121.9 kJ��mol-1��3�֣�
��2������ ��1�֣� NiOOH + H2O + e���� Ni(OH)2 + OH-��2�֣���
��3�������У�1�֣� ��H>0 ��S< 0 �����Ӧ�����Է����У�2�֣���
��

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ
 ������˵���в���ȷ����
������˵���в���ȷ����
 ����ȷ����
����ȷ����
 ˵����ȷ����
˵����ȷ����