��Ŀ����
��֪����һ��������Ϊ40%��NaOH��Һ���ܶ�Ϊ1.42g/mL����
��1����NaOH��Һ�����ʵ���Ũ��Ϊ______��
��2��ȡ����NaOH��Һ50mL����ˮϡ����355mL��ϡ�ͺ��NaOH��Һ�����ʵ���Ũ��Ϊ______��
��3����һ����������Ļ����Һ��ȡ��10mL��������BaCl2��Һ�����ˡ�ϴ�ӡ���ɺ�õ�6.99g�ij�������Һ�루2����ϡNaOH��Һ��Ӧ����ȥ35mL��Һʱǡ����ȫ�кͣ�������Һ��H2SO4��HNO3�����ʵ���Ũ�ȸ��Ƕ��٣���д��������̣�
�⣻��1�������Һȡ1ml��
����m=��v���ɵ���ȡ��Һ������=1.42��1=1.42g��
�����ʵ�����=1.42��40%=0.568g�����ʵ����ʵ���=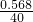 =0.0142mol��
=0.0142mol��
����c=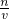 ���ɵø���Һ���ʵ���Ũ��=
���ɵø���Һ���ʵ���Ũ��= =14.2mol?L-1��
=14.2mol?L-1��
�ʴ�Ϊ��14.2 mol?L-1��
��2����Ϊ355mlϡ��Һ�������ʵ����ʵ�����50ml��Һ�������ʵ����ʵ�����ȣ�����355ml��Һ�������ʵ����ʵ���=0.05��14.2=0.71mol��
����ϡ�ͺ��NaOH��Һ�����ʵ���Ũ��= =2mol?L-1
=2mol?L-1
�ʴ�Ϊ��2.0 mol?L-1��
��3��6.99g������BaSO4����������BaSO4�����ʵ���Ϊ0.03mol��������������ʵ���Ϊ0.03mol��
����������ʵ���Ũ��= =3mol?L-1��
=3mol?L-1��
35ml��Һ������OH-�����ʵ���=0.035��2=0.07mol��
�к���ЩOH-�����H+�����ʵ���Ϊ0.07mol����������������Ļ����Һ��H+�ܵ����ʵ���Ϊ0.07mol����0.03mol����������H+�����ʵ���Ϊ0.06mol�����������ṩ��H+Ϊ0.07-0.06=0.01mol������������ʵ���Ϊ0.01mol��
����������ʵ���Ũ��= =1mol?L-1��
=1mol?L-1��
��c��H2SO4��=3.0 mol?L-1��c��HNO3��=1.0 mol?L-1
��������1���������ʵ���Ũ��=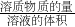 ����c=
����c=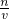 ��
��
��2����Һϡ��ǰ���������ʵ����ʵ������䣻
��3��H+��OH-1��1�кͣ������ߵ����ʵ�����Ӧ������OH-�����������H+��������H+����������Ṳͬ�ṩ�ģ���Ҫ�ȸ��ݳ��������������������Ӷ���һ��������������
����������С��һ��Ҫ��ȷ��������BaSO4����Ϊ0.03mol������SO42-�غ㣬��0.03molBaSO4�е�SO42-���������ṩ������ǽ��С���ͻ�ƿڣ�
����m=��v���ɵ���ȡ��Һ������=1.42��1=1.42g��
�����ʵ�����=1.42��40%=0.568g�����ʵ����ʵ���=
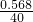 =0.0142mol��
=0.0142mol������c=
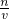 ���ɵø���Һ���ʵ���Ũ��=
���ɵø���Һ���ʵ���Ũ��= =14.2mol?L-1��
=14.2mol?L-1���ʴ�Ϊ��14.2 mol?L-1��
��2����Ϊ355mlϡ��Һ�������ʵ����ʵ�����50ml��Һ�������ʵ����ʵ�����ȣ�����355ml��Һ�������ʵ����ʵ���=0.05��14.2=0.71mol��
����ϡ�ͺ��NaOH��Һ�����ʵ���Ũ��=
 =2mol?L-1
=2mol?L-1�ʴ�Ϊ��2.0 mol?L-1��
��3��6.99g������BaSO4����������BaSO4�����ʵ���Ϊ0.03mol��������������ʵ���Ϊ0.03mol��
����������ʵ���Ũ��=
 =3mol?L-1��
=3mol?L-1��35ml��Һ������OH-�����ʵ���=0.035��2=0.07mol��
�к���ЩOH-�����H+�����ʵ���Ϊ0.07mol����������������Ļ����Һ��H+�ܵ����ʵ���Ϊ0.07mol����0.03mol����������H+�����ʵ���Ϊ0.06mol�����������ṩ��H+Ϊ0.07-0.06=0.01mol������������ʵ���Ϊ0.01mol��
����������ʵ���Ũ��=
 =1mol?L-1��
=1mol?L-1����c��H2SO4��=3.0 mol?L-1��c��HNO3��=1.0 mol?L-1
��������1���������ʵ���Ũ��=
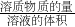 ����c=
����c=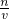 ��
����2����Һϡ��ǰ���������ʵ����ʵ������䣻
��3��H+��OH-1��1�кͣ������ߵ����ʵ�����Ӧ������OH-�����������H+��������H+����������Ṳͬ�ṩ�ģ���Ҫ�ȸ��ݳ��������������������Ӷ���һ��������������
����������С��һ��Ҫ��ȷ��������BaSO4����Ϊ0.03mol������SO42-�غ㣬��0.03molBaSO4�е�SO42-���������ṩ������ǽ��С���ͻ�ƿڣ�

��ϰ��ϵ�д�
�����Ŀ
