��Ŀ����
��13�֣�(1)��ͼ��ʾװ�ã�Ϊʵ������ʵ��Ŀ�ģ�����y���ʺ�������ڵ���_____���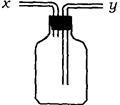
A ƿ��ʢҺ�����������Ը�������
B ƿ��ʢҺ��ϴ�Ӽ������Գ�ȥij�����е�����
C ƿ��ʢˮ�����Բ���ij������ˮ����������
D ƿ���������壬��ˮʱ����ɱ��ų�
E �ռ��ܶȱȿ����������
F �ռ��ܶȱȿ���С������
(2) ŨH2SO4��ľ̿�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽ��_______________________��
ͼ�����߿��е�װ�ÿ���������Ũ������ľ̿���ڼ��������·�Ӧ����������������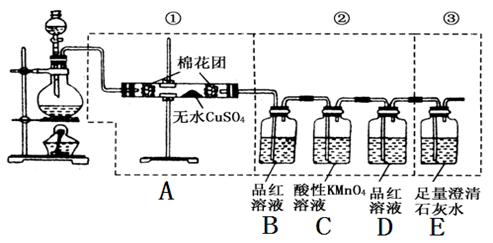
��д���пհס�
��A����ˮ����ͭ������_____________��
��֤��SO2һ�����ڵ�������_____________��
��C������KMnO4��Һ������_____________��
��֤��CO2һ�����ڵ�������_____________��
��1�� A B E (2) C+2H2SO4 CO2��+2SO2��+2H2O
CO2��+2SO2��+2H2O
�ټ���ˮ���� ��B��Ʒ����ɫ �۳�ȥSO2 ��D��Ʒ�첻��ɫ��E�б����
����

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ


