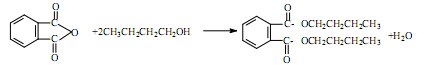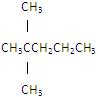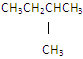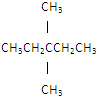��Ŀ����
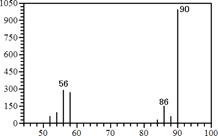
ij�л�������3.2 g�������г��ȼ��ֻ����CO2��H2O��������������ͨ��ʢ��Ũ�����ϴ��ƿ��ʢ�м�ʯ�ҵĸ���ܣ�ʵ����װ��Ũ�����ϴ��ƿ����3.6 g��ʢ�м�ʯ�ҵĸ��������4.4 g���������ж���ȷ����
| A��ֻ��̼��������Ԫ�� |
| B���϶�����̼���⡢������Ԫ�� |
| C���϶�����̼����Ԫ�أ����ܺ�����Ԫ�� |
| D��������Ŀ������������л�������ʽ����������л���ķ���ʽ |
B
���������3.2g���л�����������ȼ�պ����ɵIJ�������ͨ��Ũ����ͼ�ʯ�ң��ֱ�����3.6g��4.4g������ˮ��������3.6g��������ԭ�ӵ����ʵ�����0.4mol��������ԭ�ӵ����ʵ�����0.2mol�����ɵĶ�����̼��������4.4g��̼ԭ�ӵ����ʵ�����0.1mol������̼ԭ���غ㣬����֪���л����к���0.1mol̼ԭ�Ӻ�0.4mol��ԭ�ӣ����������غ㣬�μӷ�Ӧ��������������4.8g�������ʵ�����0.15mol����0.3mol����ԭ�ӣ�������ԭ���غ㣬�л������к�����ԭ��0.1mol��һ������̼Ԫ�غ���Ԫ�غ���Ԫ�أ���ѡB��

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ