��Ŀ����
��10�֣��±���Ԫ�����ڱ���һ���֣���������й����⣺
��1��д������Ԫ�ط��ţ���________________����________________����________________��
��2������ЩԪ���У�����õĽ���Ԫ����________________������õķǽ���Ԫ����________________������õ�Ԫ����?________________?��
��3������ЩԪ�ص�����������Ӧˮ�����У�������ǿ����________________��������ǿ����________________�������Ե�����������________________��д������֮�����Ӧ�Ļ�ѧ����ʽ��
��4������ЩԪ���У�ԭ�Ӱ뾶��С����________________��ԭ�Ӱ뾶������________________��
��5���ڢ�����У���ѧ���ʽϻ��õ���________________�������û�ѧʵ��֤������________________________________________________________________��
�ڢ���12�У���ѧ���ʽϻ��õ���________________�������û�ѧʵ��֤������________________________________________________________________��
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | | �� | | �� | |
| 3 | �� | �� | �� | �� | | �� | �� | �� |
| 4 | �� | 11 | | | | | 12 | |
��2������ЩԪ���У�����õĽ���Ԫ����________________������õķǽ���Ԫ����________________������õ�Ԫ����?________________?��
��3������ЩԪ�ص�����������Ӧˮ�����У�������ǿ����________________��������ǿ����________________�������Ե�����������________________��д������֮�����Ӧ�Ļ�ѧ����ʽ��
��4������ЩԪ���У�ԭ�Ӱ뾶��С����________________��ԭ�Ӱ뾶������________________��
��5���ڢ�����У���ѧ���ʽϻ��õ���________________�������û�ѧʵ��֤������________________________________________________________________��
�ڢ���12�У���ѧ���ʽϻ��õ���________________�������û�ѧʵ��֤������________________________________________________________________��
��1��N Si S ��2��K F Ar
��3��HClO4 KOH Al��OH��3 3HClO4+ Al��OH��3====Al��ClO4��3+3H2O��HClO4+KOH====KClO4+H2O��KOH+Al��OH��3====KAlO2+2H2O��KOH+Al��OH��3====K[Al��OH��4]
��4��F K
��5��Na ���������Ǹ�ˮ��Ӧ��ʵ��֤�����Ƹ�ˮ���ҷ�Ӧ���ų�������������ǿ�Mg����ˮ�ŷ�Ӧ���ų���������������ǿ��Mg��OH��2 Cl ��������ͨ���廯����Һ��ʵ��֤������Һ���غ�ɫ�������ķ�ӦΪ Cl2+2NaBr====2NaCl+Br2��
��3��HClO4 KOH Al��OH��3 3HClO4+ Al��OH��3====Al��ClO4��3+3H2O��HClO4+KOH====KClO4+H2O��KOH+Al��OH��3====KAlO2+2H2O��KOH+Al��OH��3====K[Al��OH��4]
��4��F K
��5��Na ���������Ǹ�ˮ��Ӧ��ʵ��֤�����Ƹ�ˮ���ҷ�Ӧ���ų�������������ǿ�Mg����ˮ�ŷ�Ӧ���ų���������������ǿ��Mg��OH��2 Cl ��������ͨ���廯����Һ��ʵ��֤������Һ���غ�ɫ�������ķ�ӦΪ Cl2+2NaBr====2NaCl+Br2��
�����ؼ�Ҫ���գ���1��20��Ԫ�ص����Ƽ����ţ���Ԫ�����ڱ��Ľṹ�����ܸ���Ԫ�����ڱ���Ԫ�����ʵݱ���ɽ��з������жϡ������û���Ӧ֤��A��B�Ľ�����ǿ������ѡ��A+Bn+ Am++B��ʵ�֣���֤��A��B�ķǽ�����ǿ������ѡ��A+Bn-
Am++B��ʵ�֣���֤��A��B�ķǽ�����ǿ������ѡ��A+Bn- Am-+B��ʵ�֡����磬����Ϊ��֤���ȵķǽ����Ա���ǿ��Ӧѡ��ӦH2S+Cl2====S��+2HCl����S2-+Cl2====S��+2Cl-����������Cl2+SO2+2H2O====H2SO4+2HCl��
Am-+B��ʵ�֡����磬����Ϊ��֤���ȵķǽ����Ա���ǿ��Ӧѡ��ӦH2S+Cl2====S��+2HCl����S2-+Cl2====S��+2Cl-����������Cl2+SO2+2H2O====H2SO4+2HCl��
 Am++B��ʵ�֣���֤��A��B�ķǽ�����ǿ������ѡ��A+Bn-
Am++B��ʵ�֣���֤��A��B�ķǽ�����ǿ������ѡ��A+Bn- Am-+B��ʵ�֡����磬����Ϊ��֤���ȵķǽ����Ա���ǿ��Ӧѡ��ӦH2S+Cl2====S��+2HCl����S2-+Cl2====S��+2Cl-����������Cl2+SO2+2H2O====H2SO4+2HCl��
Am-+B��ʵ�֡����磬����Ϊ��֤���ȵķǽ����Ա���ǿ��Ӧѡ��ӦH2S+Cl2====S��+2HCl����S2-+Cl2====S��+2Cl-����������Cl2+SO2+2H2O====H2SO4+2HCl��
��ϰ��ϵ�д�
 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
�����Ŀ
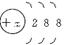
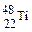 ��
��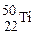 ��˵������ȷ����
��˵������ȷ���� 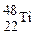 ��
��