��Ŀ����
18������˵���У���ȷ���ǣ�������| A�� | 0.1 mol•L-1KHS��Һ�У�c��K+��+c��H+��=c��OH-��+c��HS-��+c��S2-�� | |
| B�� | �����£�0.1 mol/L ��������Һ����NH4Al��SO4��2����NH4Cl����CH3COONH4������c��NH4+���ɴ�С��˳���ǣ��٣��ڣ��� | |
| C�� | ��25��ʱ����pH=11��NaOH��Һ��pH=3��CH3COOH��Һ�������Ϻ�c��Na+����c��CH3COO-����c��OH-����c��H+�� | |
| D�� | Na2CO3��Һ�У�2c��Na+��=c��CO32-��+c��HCO3-��+c��H2CO3�� |
���� A����Һ�д��ڵ���غ�����������Ӻ������������������ͬ��
B��NH4Al��SO4��2��Һ��笠�������������ˮ������ƣ�CH3COONH4��笠���������������ˮ����ٽ���
C����25��ʱ����pH=11��NaOH��Һ��pH=3��CH3COOH��Һ�������Ϻ�ٽ������ֵ��룬��Һ�����ԣ�
D��Na2CO3��Һ�д��������غ㣬n��Na��=2n��C����
��� �⣺A.0.1 mol•L-1KHS��Һ�д��ڵ���غ㣺c��K+��+c��H+��=c��OH-��+c��HS-��+2c��S2-������A����
B��NH4Al��SO4��2��Һ��笠�������������ˮ������ƣ�CH3COONH4��笠���������������ˮ����ٽ������ԣ���ͬ���ʵ���Ũ�ȵ�������Һ�У���NH4Al��SO4��2����NH4Cl����CH3COONH4����c��NH4+���ɴ�С��˳���ǣ��٣��ڣ��ۣ���B��ȷ��
C����25��ʱ����pH=11��NaOH��Һ��pH=3��CH3COOH��Һ�������Ϻ�ٽ������ֵ��룬��Һ�����ԣ�c��CH3COO-����c��Na+����c��H+����c��OH-������C����
D��Na2CO3��Һ�д��������غ㣬c��Na+��=2c��CO32-��+2c��HCO3-��+2c��H2CO3������D����
��ѡB��
���� ���⿼����������ʵ���ƽ�⡢�������Һ�е���غ㡢�����غ㡢����Ũ�ȴ�С�Ƚϵȣ�ע����Ӧ��������������ʵĵ���ƽ�⣬��Ŀ�Ѷ��еȣ�

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�| A�� | ��Ŵ��������� | |
| B�� | ʯ������Һ����ú��Һ�� | |
| C�� | �鼮�е�ֽ���ɰ�ɫ������ɻ�ɫ | |
| D�� | ������ˮ�Ե�ֲ����ά�ĸ������ˮ�Ͱ� |
| A�� | ú��Һ�� | B�� | ʯ�͵ķ��� | C�� | ʯ���ѻ� | D�� | ��ϩ�Ӿ۷�Ӧ |
| R | X | Y |
| Z | W |
| A�� | �����̬�⻯������ȶ�����ǿ����������Z��R��X��Y | |
| B�� | R��X��Y��Z��W��Ԫ����ۺ���ͼ۵ľ���ֵ֮�;�Ϊ8 | |
| C�� | ����������ˮ��������������ǿ����Z��R��W | |
| D�� | RY3��W2X��ZW5������ÿ��ԭ������㶼�ﵽ8 ���ӽṹ |
| ���� ��� | �¶ȣ��棩 | ��ʼ���ʵ�����mol�� | ƽ�����ʵ�����mol�� | |
| A��g�� | B��g�� | C��g�� | ||
| �� | 387 | 0.20 | 0.080 | 0.080 |
| �� | 387 | 0.40 | ||
| �� | 207 | 0.20 | 0.090 | 0.090 |
| A�� | �÷�Ӧ������ӦΪ��H��0 | |
| B�� | 207�棬K=4 | |
| C�� | �ﵽƽ��ʱ���������е�A����������������е���ͬ | |
| D�� | �������з�Ӧ����ƽ������ʱ����������еĶ� |
| A�� | 2Mg��s��+CO2��g����2MgO��s��+C��s�� | |
| B�� | ���������Է����еķ�Ӧ��NH3��g��+HCl��g����NH4Cl��s�� | |
| C�� | ���������Է����еķ�Ӧ��SiO2��s��+2C��s����Si��s��+2CO��g�� | |
| D�� | �κ��¶��¾����Է����еķ�Ӧ��2H2O2��l����2H2O��l��+O2��g�� |

 ��
�� ��
�� ��
�� ��
�� ��
�� ��
��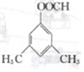 ��
�� �� �����������̣�����Ա�����ȩΪ�л�ԭ�ϣ����Լ���ѡ���Ʊ�ijҩ���м���
�� �����������̣�����Ա�����ȩΪ�л�ԭ�ϣ����Լ���ѡ���Ʊ�ijҩ���м��� �ĺϳ�·�ߣ�
�ĺϳ�·�ߣ� ��
��