��Ŀ����
ʵ������ȼ�շ��ⶨij�����ᣨCxHyNzOp���ķ�����ɡ�ȡmg ���ְ�������ڴ����г��ȼ�գ�����CO2��H2O��N2������ͼ��ʾװ�ý���ʵ�顣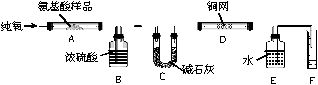
��ش��������⣺
��1��ʵ�鿪ʼʱ������Ҫͨ��һ��ʱ�����������������__________��
��2������װ������Ҫ���ȵ�������_________������ĸ��գ���ͬ��������ʱӦ�ȵ�ȼ___________���ľƾ��ơ�
��3��Aװ���з�����Ӧ�Ļ�ѧ����ʽ��_______________��
��4��װ��D��������_______________________________��
��5����ȡN2�����ʱ��Ӧע���_____________����_____________��
��6��ʵ���в��N2�����ΪVmL����״������Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������__________��
��1�����������װ���еĿ�����ʵ��������Ӱ��
��2��A��D�� D
��3��4CxHyNzOp+��4x+y��2p��O2��4xCO2+2yH2O+2zN2
��4����ȥδ��Ӧ��O2
��5������Ͳˮƽ������������ �ڶ���ʱ�������밼Һ����ʹ���ƽ
��6��B��C��װ�����ӵ������Ͱ������Ħ������
�����������

��ϰ��ϵ�д�
 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ




