��Ŀ����
���з�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ1:2���ҵ���ת����Ϊ0.1mol����
A.��֪��Cu+2Fe3+=2Fe2++Cu2+����6.4gCuƬ����0.1 mol��L��FeCl3��Һ�г�ַ�Ӧ
B. R2O82-������һ�������¿���Mn2+������MnO4������R2O82-���ӱ�ΪRO42-�����õ�����Һ�к�MnO4-0.2mol
C. C1O2��һ��ɱ����������Ч�ʸߡ�������ȾС��ˮ��������ʵ�����Ʊ�ԭ����2KClO3
+H2C2O4+H2SO4![]() 2ClO2��+K2SO4+2CO2��+2H2O��������Ƶ�����Ϊ1.12L
2ClO2��+K2SO4+2CO2��+2H2O��������Ƶ�����Ϊ1.12L
D.������ʹʪ���KI������ֽ��������ԭ��Ϊ��O3+2KI+H2O=2KOH+I2+O2�����1000mL��Һ�е�pH=13
D��
����:
A����������ΪFe3+����ԭ��ΪCu�����������뻹ԭ�������ʵ���֮��Ϊ2:1��6.4gCuʧȥ������Ϊ0.2mol�������ת����ҲΪ0.2mol��B����ÿ1mol������R2O82-��ΪRO42-�õ�2mol���ӣ�ÿ1mol��ԭ��Mn2+������MnO4��ʱ�õ�5mol���ӣ������������뻹ԭ�����ʵ���֮��Ϊ5:2������MnO4-0.2mol��ת�Ƶĵ�����Ϊ1mol��C�����������뻹ԭ�����ʵ���֮��Ϊ2:1���õ�������Ϊ0.05mol������ClO2Ϊ0.025mol��ת�Ƶĵ�����Ϊ0.025mol��D�����������뻹ԭ�����ʵ���֮��Ϊ1:2�����ɵ�KOHΪ1.0![]() 0.1mol=0.1mol������0.1molI-������ΪI2��ת�Ƶĵ�����Ϊ0.1mol��
0.1mol=0.1mol������0.1molI-������ΪI2��ת�Ƶĵ�����Ϊ0.1mol��

 �����ҵ��ٿ���������������ϵ�д�
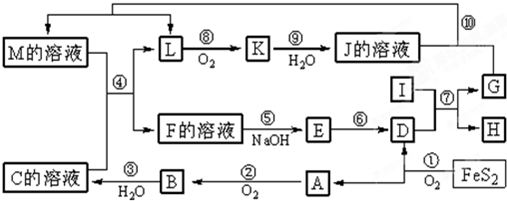
�����ҵ��ٿ���������������ϵ�д�( 8��)��ͼ������A��M��һ�������µ�ת����ϵ�����ֲ��P��Ӧ����δ�г��������У�I���ɵ�������Ԫ����ɵĵ������۵���ߵĽ����� K��һ�ֺ���ɫ���塣
|
 ����ʾ��4FeS2+11O2
����ʾ��4FeS2+11O2 2Fe2O3+8SO2��
2Fe2O3+8SO2������д���пհף�
�� �����ڱ��У���ɵ���G��Ԫ��λ�ڵ�___ _ _����_________�塣
�� �ڷ�Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ___________________��
�� �ڢڡ��ۡ��ޡ����м����ڻ��Ϸ�Ӧ�����ڷ�������ԭ��Ӧ����___________������ţ�
�� ��Ӧ�ܵ����ӷ���ʽ�ǣ�______________________________________________��
�� ��������D��KNO3��KOH���ڣ����Ƶ�һ�֡���ɫ��������Ч��ˮ��K2FeO4��������أ���ͬʱ������KNO2��H2O���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�__________________________________��


 2Fe2O3+8SO2��
2Fe2O3+8SO2��
 2Fe2O3+8SO2��
2Fe2O3+8SO2��