��Ŀ����
��ҵ�ϵġ����������ָ���ᡢ���ᡢ���ᡢ������ռ��������Щ��ҵԭ�ϵ����������й�ϵ���簱�����������ᡢ�����ԭ�ϣ���ˮ��Һ�����Ṥҵ��β�������ռ����������ɹ�������ͼ��װ��ͼ���£�
����һ�����ƴ��������ͼ
����A�ı�����Һ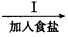 A��ʳ�εı�����Һ
A��ʳ�εı�����Һ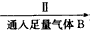 ����Һ
����Һ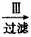 ����
����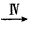 ����
����
���϶������ӽ���Ĥ����ⱥ��ʳ��ˮԭ��ʾ��ͼ
����һ�����ƴ��������ͼ
����A�ı�����Һ
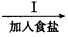 A��ʳ�εı�����Һ
A��ʳ�εı�����Һ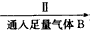 ����Һ
����Һ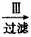 ����
���� ����
�������϶������ӽ���Ĥ����ⱥ��ʳ��ˮԭ��ʾ��ͼ

������ij������Ϊ���ۺ��������������еĸ���ƷCaSO4�������ڵĺϳɰ�����������������Ʊ�
(NH4)2SO4��������
(NH4)2SO4��������
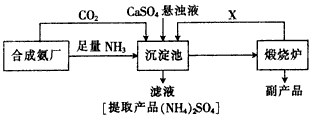
�������ϲ��ϣ��ش���������
(1)�ڢ�����������_______��Ϊʲô��ͨ��A������ͨB���壿______________��
(2)���϶��о��Ƶ�ʳ��ˮͨ�����ӽ���Ĥ���۵�⣬�������ӽ���Ĥ������a��______��b���ͨ�����Һ��______________����ⱥ��ʳ��ˮ�����ӷ���ʽ��_____________________��
(3)��ҵ�ϳ��õ�ⷨ���ռ��⣬������ʯ��--�����������д���йط�Ӧ����ʽ��_____________��
(4)�������еij������ڷ�������Ҫ��Ӧ������______________������ɫ��ѧ����Դ�ۺ����õĽǶ�˵���������̵���Ҫ�ŵ���__________________��
(1)�ڢ�����������_______��Ϊʲô��ͨ��A������ͨB���壿______________��
(2)���϶��о��Ƶ�ʳ��ˮͨ�����ӽ���Ĥ���۵�⣬�������ӽ���Ĥ������a��______��b���ͨ�����Һ��______________����ⱥ��ʳ��ˮ�����ӷ���ʽ��_____________________��
(3)��ҵ�ϳ��õ�ⷨ���ռ��⣬������ʯ��--�����������д���йط�Ӧ����ʽ��_____________��
(4)�������еij������ڷ�������Ҫ��Ӧ������______________������ɫ��ѧ����Դ�ۺ����õĽǶ�˵���������̵���Ҫ�ŵ���__________________��
(1)���ȷֽ⣻AΪ��������ͨ���ܽ�Ⱥܴ�İ�����һ�����ṩ��Ӧ���һ����ʹ��Һ�ʼ��������� CO2������
(2)Na+�����ƺ�ı���ʳ��ˮ��2Cl-+2H2O Cl2��+H2��+2OH-
Cl2��+H2��+2OH-
(3)Ca(OH)2+Na2CO3=CaCO3��+2NaOH
(4)CaSO4+CO2+2NH3+H2O=CaCO3��+(NH4)2SO4���������У�������CO2ѭ��ʹ�ã��õ��IJ�Ʒ����Ʒ�������õ����ʣ�������ɻ�����Ⱦ
(2)Na+�����ƺ�ı���ʳ��ˮ��2Cl-+2H2O
 Cl2��+H2��+2OH-
Cl2��+H2��+2OH-(3)Ca(OH)2+Na2CO3=CaCO3��+2NaOH
(4)CaSO4+CO2+2NH3+H2O=CaCO3��+(NH4)2SO4���������У�������CO2ѭ��ʹ�ã��õ��IJ�Ʒ����Ʒ�������õ����ʣ�������ɻ�����Ⱦ

��ϰ��ϵ�д�
�����Ŀ