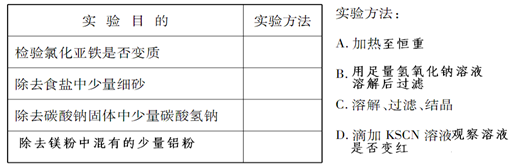
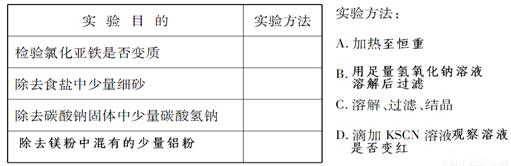
��Ŀ����
ʵ�����мס�����ƿ��ɫ��Һ������һƿ��ϡ���ᣬ��һƿ��̼������Һ��Ϊ�ⶨ�ס�����ƿ��Һ�ijɷּ����ʵ���Ũ�ȣ���������ʵ�飺
����ȡ25.00 mL����Һ�������л�����������Һ15.00 mL�����ռ���224 mL(��״��)���塣
����ȡ15.00 mL����Һ�������л����������Һ25.00 mL�����ռ���112 mL(��״��)���塣
(1)�жϣ�����________��Һ������_________��Һ��
(2)ʵ�������������Ӧ�����ӷ���ʽΪ_____________________________��
(3)����Һ�����ʵ���Ũ��Ϊ ������Һ�����ʵ���Ũ��Ϊ__________��
(4)��n mL����Һ����������Һ����������ʵ�鷽ʽ���з�Ӧ������������������ΪV mL(��״��)����V��ȡֵ��Χ��_____________________________��
(1)HCl Na2CO3
(2)CO![]() +H+ ==== HCO
+H+ ==== HCO![]() HCO
HCO![]() +H+ ==== CO2��+H2O
+H+ ==== CO2��+H2O
(3)0.8 mol��L��1 1 mol��L��1
(4)0��V��8.96n
����:
(1)���������м���Na2CO3��Һʱ��������Ӧ2H++CO![]() ==== CO2��+H2O,������ȫ��Ӧ���ټ����Na2CO3���ٷų�CO2����Na2CO3��Һ�м�������ʱ���ȷ�����ӦCO
==== CO2��+H2O,������ȫ��Ӧ���ټ����Na2CO3���ٷų�CO2����Na2CO3��Һ�м�������ʱ���ȷ�����ӦCO![]() +H+ ==== HCO
+H+ ==== HCO![]() ,������CO
,������CO![]() ��ת��ΪHCO
��ת��ΪHCO![]() ʱ���ٷ�����ӦHCO
ʱ���ٷ�����ӦHCO![]() +H+ ==== CO2��+H2O����ʵ��٢������ݿ�֪�����ļ���������ҷ�Ӧ��ʵ��ٲ�����CO2����࣬˵����ΪHCl����ΪNa2CO3��
+H+ ==== CO2��+H2O����ʵ��٢������ݿ�֪�����ļ���������ҷ�Ӧ��ʵ��ٲ�����CO2����࣬˵����ΪHCl����ΪNa2CO3��
(3)����������ʵ���Ũ��Ϊc(HCl)��Na2CO3��Һ�����ʵ���Ũ��Ϊc(Na2CO3)����ʵ��ٵã�25.00��10 �C3 L�� c(HCl) ��![]() =
=![]() ��c(HCl)=0.8 mol��L��1;
��c(HCl)=0.8 mol��L��1;
��ʵ��ڵã� 15.00��10 ��3 L��c(Na2CO3)=25.00��10��3 L��0.8 mol��L��1-![]() ,
,
c(Na2CO3)=1 mol��L��1��
(4)������ļס��Ұ�ʵ��ٵķ�ʽ����ʱ������������Ϊ��
![]() ��n��10��3 L��0.8 mol��L��1��22.4 l��mol��1 =8.96��10��3 nL,����ʵ��ڵķ�ʽ���У��ɷ�ӦCO
��n��10��3 L��0.8 mol��L��1��22.4 l��mol��1 =8.96��10��3 nL,����ʵ��ڵķ�ʽ���У��ɷ�ӦCO![]() +H+ ==== HCO-3��֪����㣬���������壬��0��V��8.96 n��
+H+ ==== HCO-3��֪����㣬���������壬��0��V��8.96 n��

 ��У����ϵ�д�
��У����ϵ�д�