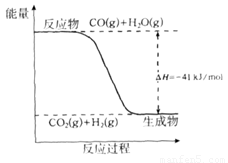
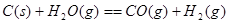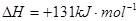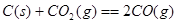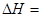��Ŀ����
�о���ѧ��Ӧ�е������仯�������ڸ��õ����û�ѧ��ӦΪ����������ȷ�����������������ǣ�������
| A������Ӧ�����жϿ���ѧ�������յ����������γɻ�ѧ�����ų����������÷�Ӧ�������ȷ�Ӧ |
| B����ͬ�����£�1gH2��O2��ȼ������Һ̬ˮ�ų�������ΪakJ����������̬ˮ���ų�������ΪbkJ����a��b |
| C��ȼ�ϵ�ȼ�ն��Ƿ��ȷ�Ӧ��ȼ����ȫȼ�շų��������Ȳ���ȫȼ�շų��������� |
| D��ȼ��ȼ�չ����зų������Ĵ�С��ȡ���ڷ�Ӧ����������������������������Դ�С |
A���ӻ�ѧ���Ƕ��жϷ�Ӧ�����Ȼ��Ƿ��ȵķ������ɼ������������������¼������ͷ���������Ӧ�����ȷ�Ӧ����֮�Ƿ��ȷ�Ӧ����A��ȷ��
B��1gH2��O2��ȼ������Һ̬ˮ�ų�������ΪakJ���ٴ�Һ̬ˮ��Ϊ��̬ˮҪ����������������ͬ�����£�1gH2��O2��ȼ��������̬ˮ�ų�������ΪbKJ��a��b����B����
C��ȼ�ϵ�ȼ�ն��Ƿ��ȷ�Ӧ��������ȫȼ�ձȲ���ȫȼ�շų���������C��ȷ��
D����Ӧ�����������������������ܣ���Ӧ�Ƿ��ȵģ���֮�������ȵģ���D��ȷ��
��ѡB��
B��1gH2��O2��ȼ������Һ̬ˮ�ų�������ΪakJ���ٴ�Һ̬ˮ��Ϊ��̬ˮҪ����������������ͬ�����£�1gH2��O2��ȼ��������̬ˮ�ų�������ΪbKJ��a��b����B����
C��ȼ�ϵ�ȼ�ն��Ƿ��ȷ�Ӧ��������ȫȼ�ձȲ���ȫȼ�շų���������C��ȷ��
D����Ӧ�����������������������ܣ���Ӧ�Ƿ��ȵģ���֮�������ȵģ���D��ȷ��
��ѡB��

��ϰ��ϵ�д�
�����Ŀ