��Ŀ����
����Ŀ���ش��������⣺
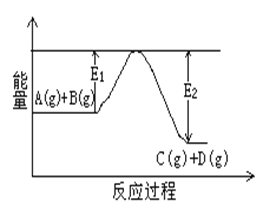
��1����ӦA(g)+B(g)![]() C(g)+D(g)�����е������仯��ͼ ��ʾ���жϸ÷�Ӧ��H___________0 (�������������������ȷ����)��
C(g)+D(g)�����е������仯��ͼ ��ʾ���жϸ÷�Ӧ��H___________0 (�������������������ȷ����)��
��2����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)= CO (g)+ H2O (g)�� ��H1= + 34.0 kJ/mol
����(g)= CO2 (g)+ H2(g) ��H2= ��7.0 kJ/mol
�����ķ���ʽΪ____________���ڸ������£���̬CO2 ����̬H2 ������̬CO����̬H2O���Ȼ�ѧ����ʽΪ __________________________________________________________________��
��3������ƽ�����ʢ��ǿ��ԭ��Һ̬��(N2H4)��ǿ������Һ̬˫��ˮ(H2O2)������0.4molҺ̬�º�0.8mol Һ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ������(�൱��25�桢101 kPa�²�õ�����)����Ӧ���Ȼ�ѧ����ʽΪ��____________________________________________________________________________��
���𰸡���CH2O2CO2 (g)+H2(g) ![]() CO(g)+H2O (g) ��H= + 41.0 kJ/molN2H4 (l)+2H2 O2 (l)
CO(g)+H2O (g) ��H= + 41.0 kJ/molN2H4 (l)+2H2 O2 (l) ![]() N2(g)+4H2O (g) ��H= -641.75 kJ/mol
N2(g)+4H2O (g) ��H= -641.75 kJ/mol
��������
��1����ͼ����Կ�����Ӧ�����������������������������÷�Ӧ������ӦΪ���ȷ�Ӧ����H��0����2�����������غ㣬�ɼ���(g)= CO (g)+ H2O (g)��֪������ķ���ʽΪCH2O2����֪���ټ��ᣨg��=CO ��g��+H2O ��g����H1=+34.0kJ/mol���ڼ��ᣨg��=CO2 ��g��+H2��g����H2=-7.0kJ/mol����̬CO2����̬H2 ������̬CO����̬H2O�Ļ�ѧ����ʽΪCO2��g��+H2��g��=CO��g��+H2O��g�����Ը��ݢ�-�ڵõ������ԡ�H=34.0kJ/mol-��-7.0kJ/mol��=+41.0kJ/mol����CO2��g��+H2��g��=CO��g��+H2O��g����H=+41.0kJ/mol����3��0.4molҺ̬�º�0.8mol Һ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ�������������Ȼ�ѧ����ʽ�����壬1mol�º�˫��ˮ֮�䷴Ӧ��ų�641.75kJ������������N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.75 kJ/mol���ʴ�Ϊ��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.75 kJ/mol��

����Ŀ��һ���¶�������1L�ܱ������м���1mol HI(g)��������Ӧ2HI(g)![]() H2(g)+I2(g)��H > 0��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��
H2(g)+I2(g)��H > 0��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��
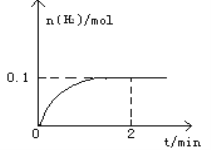
��1��2 minʱHI��ת����a(HI)=____________________�����¶��£�H2(g)+I2(g)![]() 2HI(g)��ƽ�ⳣ��K=________________��
2HI(g)��ƽ�ⳣ��K=________________��
��2���������������˵���÷�Ӧ�Ѿ��ﵽƽ��״̬��___________��
A��������ܶȲ��ٱ仯��
B���������ɫ���ٱ仯��
C���������ѹǿ���ٱ仯��
D����ͬʱ�����������������������ɵ���������
E����λʱ�����������������ĵ⻯������ʵ������
F���������������������ˡ�
��3���ں��ݾ���(������罻������)�����½���2A(g)��B(g) ![]() 2C(g)��D(s)��Ӧ���÷�Ӧ��ƽ�ⳣ���ı���ʽK=____________________,���±�����Ͷ��:
2C(g)��D(s)��Ӧ���÷�Ӧ��ƽ�ⳣ���ı���ʽK=____________________,���±�����Ͷ��:
���� | A | B | C | D |
��ʼͶ��/mol | 2 | 1 | 2 | 0 |
��Ӧ�ﵽƽ��״̬�������ϵѹǿ���ߡ��÷�Ӧ��H____0(�>������<�� ���ߡ�=��),�����÷�Ӧ��ƽ�ⳣ�����¶ȵı仯��ϵ��__________________________________________________��
��4���÷�Ӧ�����D�����ʵ�������һ�����淴Ӧ����________(���������С�� ���ߡ����䡱)��