��Ŀ����
ijѧ��������l00mL 2mol/L��NaCI��Һ��Ȼ�����ҺŨ������ȷ�ⶨ���Ҳⶨ������һ�в�������ȷ����������Һ�����ʵ���Ũ�ȵ���2mol/L����ô�����ƹ����У����в������ܵ�����ҺŨ��ƫ�͵���
��������ƽ����������NaCI���������ŷ���
�ڽ�NaCl���ձ���ϡ�ͣ�ת�Ƶ��ݻ�ΪlOOmL������ƿ�к�û��ϴ���ձ�
���ڶ���ʱ�۲�Һ�温��
�������ʱ����ˮ�����˿̶ȣ������ý�ͷ�ι���ȥ�����ˮ��ʹ��Һ��Һ��պ���̶�������
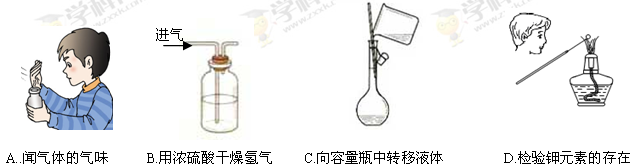
| A���ڢۢ� | B���ۢ� | C���٢ڢ� | D���٢ڢۢ� |
C
�����������������cB��nB/V�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ�����B����Һ�����V����ġ�������ʱ���ؼ�Ҫ�����ƹ�����������V�����ı仯��������һ�����ʵ���Ũ����Һʱ����nB������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����nB������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ����Ҫ�Ȼ��Ƶ�������0.1L��2mol/L��58.5g/mol��11.7g�������������ƽ����������NaCI���������ŷ��ˣ���ôʵ�ʳ������Ȼ��ƹ�������С��11.7g��������Ũ��ƫ�ͣ��ڽ�NaCl���ձ���ϡ�ͣ�ת�Ƶ��ݻ�ΪlOOmL������ƿ�к�û��ϴ���ձ��������ʼ��٣�����Ũ��ƫ�ͣ����ڶ���ʱ�۲�Һ�温�ӣ�������ƿ����Һ�����ƫ�٣�������Ũ��ƫ�ߣ��������ʱ����ˮ�����˿̶ȣ������ý�ͷ�ι���ȥ�����ˮ��ʹ��Һ��Һ��պ���̶������У������ʼ��٣�����Ũ��ƫ�ͣ����Դ�ѡC��
���㣺�������ʵ���Ũ�����Ƶ�������

�Դ�բз��һ�ּ��������ܣ��������ʫ����з������Һ�����������������������ƣ���������̨����������բз����ɫ��ɺ�ɫ��һͬѧ��Ϊ���ֺ�ɫ���ʿ��������ָʾ��һ��������������ɫ�ᷢ���ı䡣����λͬѧ�Ŀ������ԣ���Ӧ�����ڿ�ѧ̽���е�
| A��ʵ�� | B������ | C���۲� | D������ |
����ʵ�������Ҫ�õ������������в�����������ͬ����
�ٹ��� ������ ���ܽ� ��������ƿת��Һ��
| A���ٺ͢� | B���ٺ͢� | C���ۺ͢� | D���ٺ͢� |
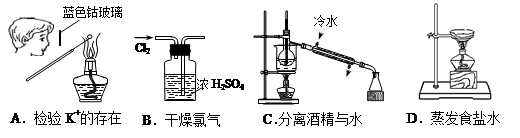
���л�ѧʵ��������¹ʴ���������ȷ���ǣ� ��
| A��Բ����ƿ����ֱ���þƾ��Ƽ��� |
| B��������Ũ����Һմ��Ƥ���ϣ�Ҫ�����ô���ˮ��ϴ��Ȼ��Ϳ������ |
| C���ƾ����Ż�ʱ����ˮ���� |
| D������������Һʱ����������Ͳ�м���һ�������ˮ�����ڽ�����������������Ũ���� |
����ʵ���������ȷ���ǣ� ��
| A����������ʱ��Ӧʹ������е�ˮ����ȫ���ɺ���ֹͣ���� |
| B������ʱ���¶ȼ�ˮ����������ƿ��֧�ܿڴ�, ����ȴˮ�������ܵ��Ͽ�ͨ���¿����� |
| C����Һ����ʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| D����1 mol��L��1�Ȼ�����Һ�м���������NaOH��Һ��ȡ������������ |
���л�ѧҩƷ����Σ�ջ�ѧƷͼ�α�־��һ�µ�һ����
| A���ռ��1 | B�����顪��2 | C���ƾ�����3 | D�����ס���4 |
����ʵ��װ�������ȷ�����ܴﵽĿ�ĵ���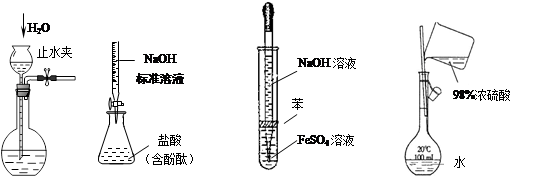
�� �� �� ��
| A��ʵ��I�� ���װ�õ������� |
| B��ʵ��II���ⶨδ֪�����Ũ�� |
| C��ʵ��III����ȡ���۲�Fe(OH)2���� |
| D��ʵ��IV������һ�������ʵ���Ũ�ȵ�ϡ������Һ |