��Ŀ����
����ͼ��ʾ��A��K������ѧ��ѧ�г������ʡ�A��һ���ᣬE��F��Iͨ��״���������壬����E�д̼�����ζ��X��Y�dz����Ľ������ʣ�JΪ���ɫ�������μӷ�Ӧ��ˮ�Լ����ɵ�ˮ��ͼ�о�����ȥ��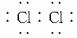
������������⣺
��1��E�ĵ���ʽΪ____________________��J�Ļ�ѧʽΪ_____________________��
��2��д��K��Һ��Y��Ӧ�����ӷ���ʽ____________________________________________��
��3��д��D��Һ��X��Ӧ�����ӷ���ʽ_________________________________________��
��4��д��A+B![]() C+E�Ļ�ѧ����ʽ______________________________________��
C+E�Ļ�ѧ����ʽ______________________________________��
��5�����n��A����n��B��=6��1����B���ܵĻ�ѧʽ____________��
��1��?![]() ?? Fe��OH��3
?? Fe��OH��3
��2��Fe+2Fe3+====3Fe2+
��3��2Al+2OH-+2H2O====2![]() +3H2��
+3H2��
��4��2HCl+NaClO====NaCl+Cl2��+H2O
��5��NaClO3
�����������ƶ����JΪFe��OH��3��EΪ�д̼������壬YΪ�������Ƴ�EΪCl2��YΪFe��KΪFeCl3��HΪFeCl2��

��֪X��Y��ZΪ�����ɶ�����Ԫ�ع��ɵ�10�����ӵ����ӣ���ṹ�ص����£�
|
���Ӵ��� |
X |
Y |
Z |
|
ԭ�Ӻ��� |
���� |
���� |
�ĺ� |
|
���ӵĵ���� |
һ����λ����� |
һ����λ����� |
0 |
����A��X��Y���ɣ� B��C��D��K���ǵ��ʣ����й�����֮������Ӧת����ϵ����ͼ��ʾ����Ӧ�١��ݶ������ڹ�ҵ�����ķ�Ӧ����Ӧ���и��������ͼ����ȥ������д���пհף�
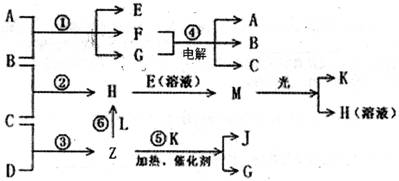
(1)Y�Ļ�ѧʽ��_______��Z���ӵĿռ乹��Ϊ_______��A�ĵ���ʽΪ_______��
(2)д�����з�Ӧ�����ӷ�Ӧ����ʽ��
H+E(��Һ)��M________________________________________________________
F+G��A+B+C_____________________________________________
(3)L����3��Ԫ�ع��ɵķ��ӣ�����Z��1��2�����ʵ���֮�ȷ�Ӧ��������CO(NH2)2������H��д��L�Ľṹʽ��____________��
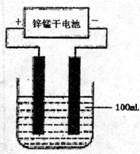
(4)�����£�ʵ����ģ�ҵ��Ӧ�ܵĹ�������ͼ��ʾ������£����缫�ϲ���112mL(������Ϊ��״���µ����)B����ʱ���ձ�����Һ��pH=______��(����������ȫ�ݳ�����Һ�������)
��֪X��Y��ZΪ�����ɶ�����Ԫ�ع��ɵ�10�����ӵ����ӣ���ṹ�ص����£�
| ���Ӵ��� | X | Y | Z |
| ԭ�Ӻ��� | ���� | ���� | �ĺ� |
| ���ӵĵ���� | һ����λ����� | һ����λ����� | 0 |
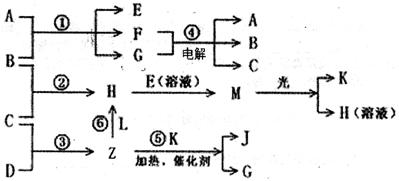
����A��X��Y���ɣ� B��C��D��K���ǵ��ʣ����й�����֮������Ӧת����ϵ����ͼ��ʾ����Ӧ�١��ݶ������ڹ�ҵ�����ķ�Ӧ����Ӧ���и��������ͼ����ȥ������д���пհף�
(1)Y�Ļ�ѧʽ��_______��Z���ӵĿռ乹��Ϊ_______��A�ĵ���ʽΪ_______��
(2)д�����з�Ӧ�����ӷ�Ӧ����ʽ��
H+E(��Һ)��M________________________________________________________
F+G��A+B+C_____________________________________________
(3)L����3��Ԫ�ع��ɵķ��ӣ�����Z��1��2�����ʵ���֮�ȷ�Ӧ��������CO(NH2)2������H��д��L�Ľṹʽ��____________��
(4)�����£�ʵ����ģ�ҵ��Ӧ�ܵĹ�������ͼ��ʾ������£����缫�ϲ���112mL(������Ϊ��״���µ����)B����ʱ���ձ�����Һ��pH=______��(����������ȫ�ݳ�����Һ�������)
��֪X��Y��ZΪ�����ɶ�����Ԫ�ع��ɵ�10�����ӵ����ӣ���ṹ�ص����£�
| ���Ӵ��� | X | Y | Z |
| ԭ�Ӻ��� | ���� | ���� | �ĺ� |
| ���ӵĵ���� | һ����λ����� | һ����λ����� | 0 |
(1)Y�Ļ�ѧʽ��_______��Z���ӵĿռ乹��Ϊ_______��A�ĵ���ʽΪ_______��
(2)д�����з�Ӧ�����ӷ�Ӧ����ʽ��
H+E(��Һ)��M________________________________________________________
F+G��A+B+C_____________________________________________
(3)L����3��Ԫ�ع��ɵķ��ӣ�����Z��1��2�����ʵ���֮�ȷ�Ӧ��������CO(NH2)2������H��д��L�Ľṹʽ��____________��
(4)�����£�ʵ����ģ�ҵ��Ӧ�ܵĹ�������ͼ��ʾ������£����缫�ϲ���112mL(������Ϊ��״���µ����)B����ʱ���ձ�����Һ��pH=______��(����������ȫ�ݳ�����Һ�������)
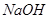 ��Һ���ܡ����������е�
��Һ���ܡ����������е�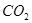 ��
��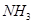 Ϊԭ�Ͽɺϳɻ�������[
Ϊԭ�Ͽɺϳɻ�������[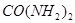 ]����֪��
]����֪��
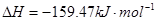 ��
��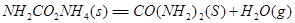
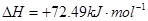 ��
��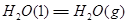
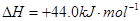 ��
��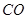 ���ڴ���������CO��
���ڴ���������CO��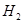 ��Ӧ���ɼ״���
��Ӧ���ɼ״���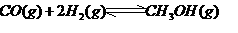 ij�ݻ��ɱ���ܱ������г���10molCO��20mol
ij�ݻ��ɱ���ܱ������г���10molCO��20mol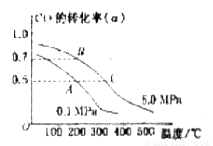
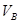 _______VL��������ڡ�����С�ڡ����ڡ���
_______VL��������ڡ�����С�ڡ����ڡ���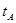 _______
_______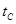 ���>������<����=����
���>������<����=����