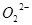��Ŀ����
һ��������Һ̬������XY2���ڱ�״���µ�һ��������O2��ǡ����ȫȼ�գ���Ӧ����ʽΪ��XY2(l)��3O2(g)===XO2(g)��2YO2(g)����ȴ���ڱ�״���²��������������672 mL���ܶ���2.56 g/L����
(1)��ӦǰO2�������________________��
(2)������XY2��Ħ��������________________��
(3)��XY2������X��Y��Ԫ�ص���������3��16����X��Y��Ԫ�طֱ�Ϊ________��__________(дԪ�ط���)����д�� Y�����ӽṹʾ��ͼΪ ��
(4)��֪XԪ����aX��bX��cX����ԭ�ӣ�YԪ����eY��f Y ����ԭ�ӣ��������ܹ��γ� ��XY2���ӡ�
(1)��ӦǰO2�������________________��
(2)������XY2��Ħ��������________________��
(3)��XY2������X��Y��Ԫ�ص���������3��16����X��Y��Ԫ�طֱ�Ϊ________��__________(дԪ�ط���)����д�� Y�����ӽṹʾ��ͼΪ ��
(4)��֪XԪ����aX��bX��cX����ԭ�ӣ�YԪ����eY��f Y ����ԭ�ӣ��������ܹ��γ� ��XY2���ӡ�
(1)672mL�� (2)76g/mol�� (3) C��S (дԪ�ط���)�� �� (4)9
�� (4)9
 �� (4)9
�� (4)9��1�����ݷ�Ӧ�ķ���ʽ��֪����Ӧǰ������Dz���ģ����Է�Ӧǰ�����������672ml��
��2����״���£�672ml��������ʵ�����0.672L��22.4L/mol��0.03mol�����μӷ�Ӧ��������0.03mol��������XY2�����ʵ�����0.01mol����״���µ��ܶ���2.56 g/L����������������2.56 g/L��0.672L�����������غ㶨�ɿ�֪��0.01��M��0.03��32��2.56 ��0.672�����M��76�����Ի�����XY2��Ħ��������76g/mol��
��3����X�����ԭ��������x����Y�����ԭ�������ǣ�76��x����2�������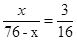 �����x��12������Y�����ԭ��������32����X��Y�ֱ���12��32�������ӵĺ����������18�������ӵĽṹʾ��ͼ��
�����x��12������Y�����ԭ��������32����X��Y�ֱ���12��32�������ӵĺ����������18�������ӵĽṹʾ��ͼ�� ��
��
��4��������ѧ��������Ͽ�֪���ܹ��γ�XY2���ӵ�������3��3��9�֡�
��2����״���£�672ml��������ʵ�����0.672L��22.4L/mol��0.03mol�����μӷ�Ӧ��������0.03mol��������XY2�����ʵ�����0.01mol����״���µ��ܶ���2.56 g/L����������������2.56 g/L��0.672L�����������غ㶨�ɿ�֪��0.01��M��0.03��32��2.56 ��0.672�����M��76�����Ի�����XY2��Ħ��������76g/mol��
��3����X�����ԭ��������x����Y�����ԭ�������ǣ�76��x����2�������
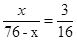 �����x��12������Y�����ԭ��������32����X��Y�ֱ���12��32�������ӵĺ����������18�������ӵĽṹʾ��ͼ��
�����x��12������Y�����ԭ��������32����X��Y�ֱ���12��32�������ӵĺ����������18�������ӵĽṹʾ��ͼ�� ��
����4��������ѧ��������Ͽ�֪���ܹ��γ�XY2���ӵ�������3��3��9�֡�

��ϰ��ϵ�д�
�����Ŀ