��Ŀ����
17���������ư�ȫ��������һ����Ҫ�Ļ�ѧ���ʣ��������������й㷺������������ɱ��������������������֬Ư���ȣ���ͼ�ǹ�ҵ����ʯ��ʯ��˫��ˮΪԭ�������������Ƶ�ʵ�����̣����У�ʯ��ʯ�ﺬ��һ���������������ʣ�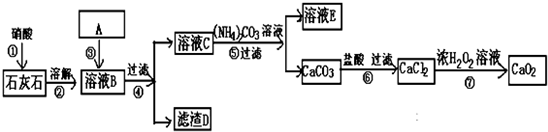
��1�����A��һ����Һ�������Һ������NH3•H2O��Һ��NaOH��Һ������A��Ŀ���dz�ȥ���е�����Fe3+
��2������ܺͲ���IJ������õ��IJ���������©�����ձ���������
��3����������ڵ����µĹ�������Ũ��Һ�м�����ˮ�Ȼ��ƣ�һ��ʱ����ټ�������������������Һ����ʱ������ִ����Ĺ������Ƴ�����д���ù����з�����Ӧ���ܵĻ�ѧ����ʽ��CaCl2+H2O2+2NaOH=CaO2+2NaCl+2H2O
��4����c mol•L-1KMnO4��Һ�ζ�a g��������������Һ�����ζ��յ�ʱ����V mL KMnO4��Һ������������������Һ�й������������������$\frac{8.5cV}{a}$
��5����ҵ�����У��Ʊ���CaO2ͨ�������������ȵĽᾧˮ���仯ѧʽΪCaO2•XH2O��������CaO���ʣ���ȡ0.542g��Ʒ���ȣ�������Ӧ��2CaO2•XH2O�T2CaO+O2+2XH2O���õ������O267.2ml��������ͬ������Ʒ����ϡ���ᣬ��������Na2CO3��Һ���õ�0.70g�����������X=0.5��
���� ʯ��ʯ�ﺬ��һ���������������ʣ�ʯ��ʯ���������ܽ�õ��������Һ������AΪ���������ӵ��Լ����������������ƻ�ˮ��Һ�����˵õ���Һ����Ҫ�Ǹ����ӣ����� ̼�����Һ����̼��Ƴ��������˵õ�̼��ƣ��������ܽ������Ȼ�����Һ����Ũ�Ĺ���������Һ���ɹ������ƣ�
��1��A�dz��������ӵ��Լ���
��2������ܺͲ���IJ����ǹ��ˣ���ϲ���ѡ������
��3����������ڵ����µĹ�������Ũ��Һ�м�����ˮ�Ȼ��ƣ�һ��ʱ����ټ�������������������Һ����ʱ������ִ����Ĺ������Ƴ���������ԭ���غ���ƽ��д��ѧ����ʽ��
��4�����ݸ�����غ������ⷴӦ�Ķ�����ϵ���㷴Ӧ�Ĺ������⣬����õ�����������
��5������CaCO3�����������ɵ���Ʒ��CaԪ�ص�����������CaԪ�������غ����CaO����������������ȥCaO2��CaO����������ˮ����������������H2O��CaO2�����ʵ�����ֵ�����ɵõ�x��ֵ��
��� �⣺ʯ��ʯ�ﺬ��һ���������������ʣ�ʯ��ʯ���������ܽ�õ��������Һ������AΪ���� �����ӵ��Լ����������������ƻ�ˮ��Һ�����˵õ���Һ����Ҫ�Ǹ����ӣ����� ̼�����Һ����̼��Ƴ��������˵õ�̼��ƣ��������ܽ������Ȼ�����Һ����Ũ�Ĺ���������Һ���ɹ������ƣ�
��1��ʯ��ʯ�ﺬ��һ���������������ʣ��ܽ���������ӣ�����A�dz��������ӵ��Լ���Ӧѡ��ˮ��Һ������������Һ��Ŀ���dz�ȥ���е�����Fe3+��
�ʴ�Ϊ��NH3•H2O��Һ��NaOH��Һ����ȥ���е�����Fe3+��
��2������ܺͲ���IJ����ǹ��ˣ���ϲ���ѡ��������Ϊ��©�����ձ������������ʴ�Ϊ��©�����ձ�����������
��3����������ڵ����µĹ�������Ũ��Һ�м�����ˮ�Ȼ��ƣ�һ��ʱ����ټ�������������������Һ����ʱ������ִ����Ĺ������Ƴ���������ԭ���غ���ƽ��д��ѧ����ʽΪCaCl2+H2O2+2NaOH=CaO2+2NaCl+2H2O��
�ʴ�Ϊ��CaCl2+H2O2+2NaOH=CaO2+2NaCl+2H2O��
��4�����ݸ�����غ������ⷴӦ�Ķ�����ϵ���㷴Ӧ�Ĺ������⣬
5H2O2+2MnO4-+6H+=5O2��+8H2O+2Mn2+��
5 2
n V L��c mol•L-1
n=2.5cVmol
����õ�����������������=$\frac{2.5cVmol��34g/mol}{ag}$��100%=$\frac{8.5cV}{a}$��
�ʴ�Ϊ��$\frac{8.5cV}{a}$��
��5����Ӧ�����ɱ����O267.2ml��0.003mol�����ݷ�Ӧ2CaO2•XH2O�T2CaO+O2+2XH2O����֪n��CaO2•xH2O��=0.006mol����Ϊn��CaCO3��=$\frac{0.7}{100}$mol=0.007mol������CaԪ���غ㣬��֪��n��CaO��=0.007mol-0.006mol=0.001mol��m��CaO��=0.001mol��56g/mol=0.056g����Ʒ��ˮ������Ϊ��m��H2O��=0.542g-m��CaO2��-m��CaO��=0.542g-0.006mol��72g/mol-0.056g=0.054g��n��H2O��=$\frac{0.054g}{18g/mol}$=0.003mol��
x=$\frac{0.003mol}{0.006mol}$=0.5��
�ʴ�Ϊ��0.5��
���� ���⿼�������ʷ�����ᴿ�ķ��������̷����жϣ�ʵ�����������ѧ����ʽ����Ӧ�ã����������Ϣ������Ŀ���ǹؼ�����Ŀ�Ѷ��еȣ�

| ����̿��mol�� | NO��mol�� | CO2��mol�� | N2��mol�� | |
| ��ʼ״̬ | 3.0 | 0.8 | 0 | 0 |
| 2minʱ | 2.8 | 0.4 | 0.2 | 0.2 |
a����λʱ��������2mol NO��ͬʱ����1mol N2
b��NO���ʵ�����CO2���ʵ���֮��Ϊ2��1
c�������л�������ѹǿ����
d�����������ܶȲ���
��2��0��2min�ڣ���NO��ʾ�÷�Ӧ��ƽ�����ʦԣ�NO��=0.1mol?L-1?min-1��
��3����֪�����¶ȣ��ÿ��淴Ӧ�Ļ�ѧƽ�ⳣ��K������÷�Ӧ�����ȷ�Ӧ������ȡ����ȡ�����T��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=9/16����2minʱ����Ӧ���ʴ��ڣ�����ڡ��������ڡ���С�ڡ����淴Ӧ���ʣ����÷�Ӧ�ﵽƽ��ʱ��NO��ƽ��ת����Ϊ60.0%��
��4���������������䣬�ı��������������ý�����ȷ����a��b������ĸ��ţ���
a����ѹʱ���������г��뺤������Ӧ���ʼ�С
b�������¶ȣ���ѧƽ�������ƶ�
c������NO��Ũ�ȣ�NO��ת��������
d����С�����������ƽ�ⳣ������
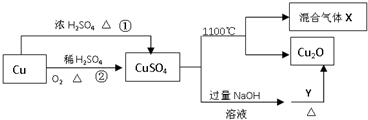
| A�� | ;���ٺ�;���ڶ���������������Ժ������� | |
| B�� | Y�����Ǿƾ���Һ | |
| C�� | CuSO4��1100��ֽ���������X������SO2��SO3�Ļ������ | |
| D�� | ��CuSO4��Һ����Ũ������ȴ�ᾧ�����Ƶõ������� |
| A�� | ��Һ�е������ӵ���Ŀ���� | |
| B�� | ��ֵ$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$���� | |
| C�� | ����ĵ���̶�����c��H+�������� | |
| D�� | �ټ���10mL pH=11��NaOH��Һ�����ҺpH=7 |
��������Һ ��������Ȼ�̼��Һ ���Ȼ�����Һ ��̼������Һ��
| A�� | ����� | B�� | ����� | C�� | ����� | D�� | ����� |
�仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���ʾ��
| T���棩 | 700 | 800 | 830 | 1 000 | 1 200 |
| K | 2.6 | 1.7 | 1.0 | 0.9 | 0.6 |
| A�� | a��0 | |
| B�� | �ɲ���������ѹ�仯���ж���ѧ��Ӧ�Ƿ�ﵽƽ�� | |
| C�� | �¶�Ϊ830��ʱ����c��CO2��•c��H2����c��CO��•c��H2O������ʱ��δ��ƽ�� | |
| D�� | �¶Ȳ��䣬����c��CO2����ƽ�����ƣ�K���� |
 ��B��������Al�����أ�Ga������������Ԫ�أ��ڢ�A�壩�����ǵĻ�������ʶ�����Ҫ��;���ش��������⣺
��B��������Al�����أ�Ga������������Ԫ�أ��ڢ�A�壩�����ǵĻ�������ʶ�����Ҫ��;���ش��������⣺ ��
�� ij�����A������Al2��SO4��3��Al2O3��Fe2O3����һ���������¿�ʵ����ͼ��ʾ�ı仯����ش��������⣺
ij�����A������Al2��SO4��3��Al2O3��Fe2O3����һ���������¿�ʵ����ͼ��ʾ�ı仯����ش��������⣺