��Ŀ����
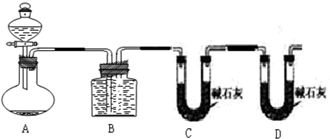
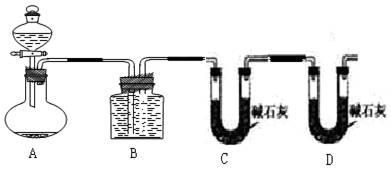
�ס�������ʵ��С��ֱ���С�Na2CO3��NaCl�������Na2CO3�����IJⶨ����ʵ�飺(1)�����ó�������������һ�����Ļ�����ܽ�����������CaCl2,Ȼ����ˣ��������ù���װ�ò���ʱ����Ҫ���е�һ�������____________________________��������ʵ������У�ʹ����ƽ����Ҫ_______�Ρ�
(2)�������������������һ�����Ļ���������������ᷴӦ��Ȼ����ͼ2-1-3��ʾװ�òⶨ������CO2������������������ƿ�е���Һ��_______����װ�òⶨ����������Ƿ�ȷ��˵�����ɣ�__________________________________________��
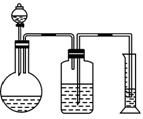
ͼ2-1-3
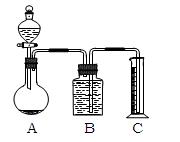
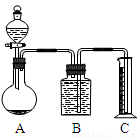
(3)���������һ����ס����������ͬ��ʵ�鷽�����ⶨ������е�Na2CO3������������____________________________���õ�����Ҫ�Լ���_______ (��������)���õ���������Ҫ��_______________________________________________________________(��������)��
������(1)��Ϊ�������õ��Ĺ�������ϸ�����Һ��ijЩ���ӣ���Ҫ��ˮ������ϴ��2��3�Ρ�Ҫ�ⶨ�������̼���Ƶĺ�������֪������������������̼��Ƶ���������̼��������Ļ����Ҫ���������γ�����������0.1 g����ʹ����ƽ�Ĵ�������Ҫ3�Ρ�
(2)���ڶ�����̼����ˮ�Ҵ���ƽ�⣺
CO2+H2O![]() H2CO3
H2CO3![]() H++
H++![]() Ϊ���ٶ�����̼���ܽ��������ƿ�пɼ��뱥��NaHCO3��Һ�������ڷ�Ӧ�����Ķ�����̼�л���������HCl���壬��NaHCO3��Ӧ����������̼���������ø�װ�òⶨ�Ķ�����̼����Dz�ȷ�ġ�
Ϊ���ٶ�����̼���ܽ��������ƿ�пɼ��뱥��NaHCO3��Һ�������ڷ�Ӧ�����Ķ�����̼�л���������HCl���壬��NaHCO3��Ӧ����������̼���������ø�װ�òⶨ�Ķ�����̼����Dz�ȷ�ġ�
(3)��Ϊ̼������Һ�����ᷴӦ�����Ȼ����������Ӧ�����Կ��ñ�������Һ�ζ�һ�������Ļ�����������������Һ��������ⶨ̼���Ƶĺ�����
�𰸣�(1)������ϴ�� 3��
(2)����NaHCO3��Һ ��ȷ������HCl��NaHCO3��Ӧ������CO2
(3)�кͷ�����(���к͵ζ��������ñ�������Һ�ζ�һ�����Ļ������Һ) ��������Һ������ �ζ��ܡ���ƿ

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�