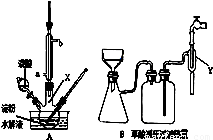
��Ŀ����
��������������ˮ��IJ��C6H12O6�����Ƶ��������ᣬװ����ͼA��ʾ�����ȡ�����������̶�װ�þ�����ȥ������֪������������ˮ��Һ�����пɷ������з�Ӧ��
C6H12O6��12HNO3��3H2C2O4��9NO2����3NO����9H2O
C6H12O6��8HNO3��6CO2��8NO����10H2O
3H2C2O4��2HNO3��6CO2��2NO����4H2O����
ʵ��������£�
��1��1�ĵ���ˮ��Һ����������(98%)�����ձ��У�
ˮԡ������85�桫90�棬����30 min��Ȼ�����¶Ƚ���60�����ң�
�ڽ�һ�����ĵ���ˮ��Һ��������X�У�
�ۿ��Ʒ�ӦҺ�¶���55��60�������£��߽�������μ�һ�����������������Ļ��ᣨ65%HNO3��98%H2SO4��������Ϊ2��1.5����Һ��
�ܷ�Ӧ3h���ң���ȴ����ѹ���˺����ؽᾧ�ò��ᾧ�塣
��ش��������⣺
��1������X�����ƣ� ��
��2��������ˮ�Ľ����� ����a��b����������������������Ҫ������������������������
��3��ʵ����������μӹ��죬�����²�������½�����ԭ���� ��
��4����װ������һ��ȱ��������������������������������������������������������
��5�������ؽᾧ�ļ�ѹ����װ����ͼB������Y����ˮ��ͷ�ϣ���������������������������
��ѹ���˵IJ����У��ٽ����������Һ����©�����ڽ���ֽ����©������ˮʪ�۴�ˮ��ͷ���ܹر�ˮ��ͷ���ݲ�����Ƥ�ܡ���ȷ��˳����������������������������
(6) ����Ʒ�ں�������Լ90�����º�������أ��õ���ˮ�ϲ��ᡣ��KMnO4����Һ�ζ����÷�Ӧ�����ӷ���ʽΪ��
2MnO4���� 5H2C2O4 �� 6H+ = 2Mn2+�� 10CO2���� 8H2O
��ȡ����Ʒ0.12 g��������ˮ��ȫ�ܽ⣬Ȼ����0.020 mol��L��1
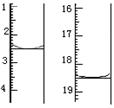
������KMnO4��Һ�ζ����յ㣨���ʲ����뷴Ӧ������ʱ��Һ
��ɫ�� ��Ϊ ���ζ�ǰ��ζ����е�Һ�������ͼ��
ʾ����ò��ᾧ����Ʒ�ж�ˮ�ϲ������������Ϊ ��
(1) ������ƿ��2��
(2) a ��1�� ���ᡭ1��
(3) �¶ȹ��ߡ�����Ũ�ȹ�����H2C2O4��һ�������� ��2�֡�
(4)ȱ��β������װ�á�2��
(5)��������ã�ʹ����ƿ����ȫƿ�е�ѹǿ��С����2�֡����ڢۢ٢ݢܡ�2��
����ɫ �Ϻ�ɫ������ɫ����1�� 84.0% ��2��
����

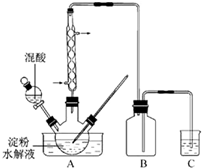 ��2011?���գ�������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ��
��2011?���գ�������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ�� ������Yԭ�ӹ�����ӻ�����Ϊ ��1mol
������Yԭ�ӹ�����ӻ�����Ϊ ��1mol ������ĿΪ ��
������ĿΪ �� �ķе�Ȼ�����
�ķе�Ȼ����� �ĸߣ�����Ҫԭ����
�ĸߣ�����Ҫԭ����  ��
�� ����Ӧ�Ļ�ѧ����ʽΪ ��
����Ӧ�Ļ�ѧ����ʽΪ ��