��Ŀ����
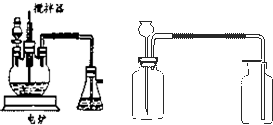
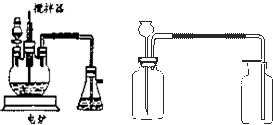
��ҵ����������豸��Ϊ���֣�һ�Ƿ���¯�����ǽӴ��ҡ����������������չ�ҵ���Ʊ������������Ƴ������װ�ã���̽���й����⡣
��ش��������⣺
��1��д��FeS2��������Ӧ�Ļ�ѧ����ʽ��____________________________��
��2������װ���г�����������__________________________________________��
��3��д����ͼ��װ�õ�����__________________________________________��
��4����װ���ҵķ�Ӧ����Ҫ����������Ҫ�ȶ������������һ�����ң�ʵ��ʱ������ο�������Ƶģ�____________________________��
��5���Ӵ�������������У�����Ӧ�ȶ�δ�����ã���ÿ����1 ![]() 2SO3��g������H=-98.3 kJ��mol-1�������������������еõ�������ã�������Ӧ�Ȳ��ƣ�����ÿ����1
2SO3��g������H=-98.3 kJ��mol-1�������������������еõ�������ã�������Ӧ�Ȳ��ƣ�����ÿ����1
��6����ʵ�����Ӷ�װ�õ�Ŀ����Ϊ��̽��____________________________��
��7����ʵ����ƻ����ڵĽ�����ȱ����_______________________________��
����������¯�ڷ�ӦΪ4FeS2+11O2![]() 2Fe2O3+8SO2��
2Fe2O3+8SO2��
����SO2��O2ת��ΪSO3�ķ�Ӧ�ǿ��淴Ӧ������������ӹ��棻ע�ҵ����SO3��ԭ���Dz���Ũ���ᣬ˵��SO3����Ũ������ü�װ�ÿ��������������������巴Ӧ������ķ����dz����ķ�������ͨ����������A��B���۲�װ����ð�������ʿ���������������Ӧ�Ⱥͷ�Ӧ���ʵ��������ȣ�98.3 kJ��mol-1��5 mol-360 kJ=131.5 kJ��
ע��Աȡ���������װ�õ������Լ���ͬ�͵��ܳ��̲�ͬ����֪��Ϊ����SO3װ�ã���Ϊ�Ա�װ�ã�������ʶ�ͽ�Լ��ʶ�����������ǻ�ѧʵ����뿼�ǵ����⣬ȱ��β������װ�á�
�𰸣�
��1��4FeS2+11O2![]() 2Fe2O3+8SO2
2Fe2O3+8SO2
��2��SO2��O2��SO3
��3������SO3���壨ģ����������
��4������ͨ���������������������������۲��װ�õ�ð�����ʹ���
��5��131.5
��6��̽����ҵ��Ϊ�β���98.3%��Ũ���������������������ˮ�����ղ���������
��7��ȱ��β������װ��

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�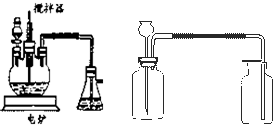 ����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�
����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�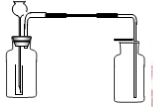
 ����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�
����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�