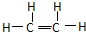��Ŀ����
��14�֣���֪��ϩ�ܷ�������ת����ϵ��
�Իش��������⣺
��1����ϩ�ĵ���ʽΪ ��B�к������������� ��
��2��д����Ӧ�Ļ�ѧ����ʽ
�� ����Ӧ���ͣ� ��
�� ����Ӧ���ͣ� ��
�� ����Ӧ���ͣ� ��
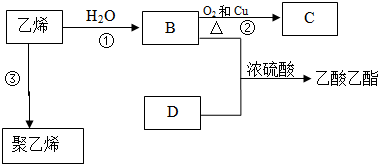
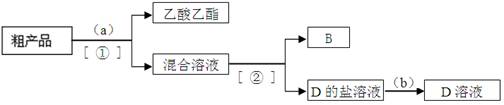
��3��������뺬B��D��ˮ�����������ֲ�Ʒ����ͼ�Ƿ���������̣�����ͼ��Բ������
�����ʵ����Լ����ڷ������������ʵ��ķ��뷽����
�Լ�a��________ __��b��_______________��
���뷽������__________������______________��
��4����B��D��Ũ����������·�����Ӧ���ƣ�B��ͬϵ��XҲ�ܺ�D������Ӧ������Y��Y�ķ�����������������28����X�ķ���ʽΪ ,X�Ľṹ��ʽΪ ��д��һ�ּ��ɣ���
��14�֣���1�� 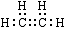 �ǻ� ����1�֣�
�ǻ� ����1�֣�
��2��������ʽ��1�֣���Ӧ����1�֣�
��CH2=CH2+H2O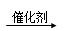 CH3CH2OH �ӳɷ�Ӧ
CH3CH2OH �ӳɷ�Ӧ
��2CH3CH2OH+O2
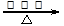 2 CH3CHO+2H2O
������Ӧ
2 CH3CHO+2H2O
������Ӧ
��CH3CH2OH+CH3COOH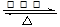 CH3COOCH2CH3+H2O
������ȡ����Ӧ
CH3COOCH2CH3+H2O
������ȡ����Ӧ
��3������̼������Һ Ũ���ᣨ�����������ȣ� ��Һ ���� ����1�֣�
��4��C4H10O ��1�֣� CH3CH2CH2CH2OH ��1�֣������𰸾����֣�
��������
�����������1����ϩ�����к���̼̼˫�����ܺ�ˮ�����ӳɷ�Ӧ�����Ҵ�����B���Ҵ����Ҵ���������������C������C����ȩ���Ҵ������ᷢ��������Ӧ����������������D��������������ϩ�к��м��Լ��ͷǼ��Լ�������ʽ��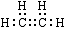 ���Ҵ��еĹ��������ǻ���
���Ҵ��еĹ��������ǻ���
��2��������ϩ�ļӳɷ�Ӧ������ʽ��CH2=CH2+H2O CH3CH2OH��
CH3CH2OH��
�����Ҵ���������Ӧ������ʽ��2CH3CH2OH+O2  2 CH3CHO+2H2O��
2 CH3CHO+2H2O��
����������Ӧ������ʽ��CH3CH2OH+CH3COOH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��3�����ɵ����������к���������Ҵ���������������������ˮ�����Կ��Լ��뱥��̼������Һ����Һ�õ����������������Һ�к��й�����̼���ơ��Ҵ��������ơ��Ҵ���ˮ�ǻ��ܵģ�����ͨ������õ��Ҵ���Ҫ�õ������ƣ�����Ҫ����Ũ�������ý�ǿ����ȡ�����������õ����ᡣ
��4��Y�ķ�����������������28����X���Ҵ���28������XӦ���Ƕ���������ʽ��C4H10O���������ܵĽṹ��ʽ��CH3CH2CH2CH2OH��CH3CH(CH3)CH2OH��CH3CH2CHOHCH3��(CH3)3COH��
���㣺���������������Ʊ������ʵķ�����ᴿ��ͬ���칹����жϵ�
�������������л��е����ʳ�ȥ����ô�������ᴿ���������һ��IJ�ͬ���ʱ˴˷ֿ����õ���Ӧ��ֵĸ�������з��롣�ڽ�����ʷ����ᴿ����ʱ,ѡ���Լ���ʵ���������Ӧ��ѭ����ԭ��: 1.���������µ����ʣ�ˮ���⣩���������ᴿ�������Ӧ�Ǵ����������Һ�����������������ʻ������У�2.�����ᴿ�������״̬���䣻3.ʵ����̺Ͳ������������У���ѡ������ᴿ����Ӧ��ѭ��������ѧ���ȼ��ӵ�ԭ��