��Ŀ����
����Ŀ����֪��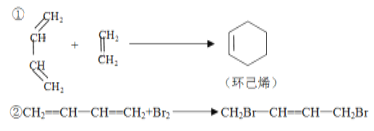
(1)д�����з�Ӧ����Ľṹ��ʽ��
H2C=CHCH=CHCH3+H2C=CHCHO�� _______________��
(2)��ij����AΪ��ʼԭ�Ϻϳɻ�����G ��·������(ͼ�� Mr ��ʾ��Է�������)
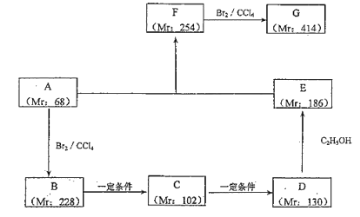
��д����Ӧ���� B��C��_______��F��G��_______��
��д���������ʵĽṹ��ʽ��A��________��F��_______��
��д�����з�Ӧ�Ļ�ѧ����ʽ��
B��C��________________��D��E��_______________��
��д�� G ������������Һ��Ӧ�ķ���ʽ____________________��
���𰸡�
(1)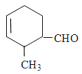 ��
�� ��
��
(2)��ˮ�ⷴӦ(ȡ����Ӧ)���ӳɷ�Ӧ��
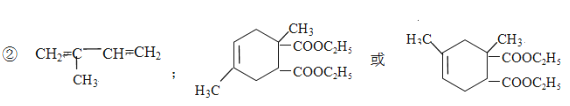 ��
��
��BrCH2-C(CH3)=CH-CH2Br+2NaOH![]() CH2(OH)-C(CH3)=CH-CH2(OH)+2NaBr��
CH2(OH)-C(CH3)=CH-CH2(OH)+2NaBr��
HOOC-C(CH3)=CH-COOH+2CH3CH2OH![]() H5C2OOC-C(CH3)=CH-COOC2H5+2H2O
H5C2OOC-C(CH3)=CH-COOC2H5+2H2O
��
��������
���������(1)������Ϣ��H2C=CHCH=CHCH3+H2C=CHCHO�� ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
(2)ij����A����Է�������Ϊ68���������̼ԭ�������ĿΪ![]() =5��8�����Ը����ķ���ʽΪC5H8���䲻���Ͷ�=
=5��8�����Ը����ķ���ʽΪC5H8���䲻���Ͷ�=![]() =2���������Ȳ�����ϩ����A���巢���ӳɷ�Ӧ����B������A��B����Է�������֪��A������1��1�ӳɣ���A�Ƕ�ϩ��������˫��ͬʱ����һ��̼ԭ���ϲ����ȶ��ṹ����A�Ľṹ��ʽΪ��
=2���������Ȳ�����ϩ����A���巢���ӳɷ�Ӧ����B������A��B����Է�������֪��A������1��1�ӳɣ���A�Ƕ�ϩ��������˫��ͬʱ����һ��̼ԭ���ϲ����ȶ��ṹ����A�Ľṹ��ʽΪ��![]() ��Bͨ��ˮ�ⷴӦ����ԭ�ӱ�ȡ������2���ǻ�����C������D��E����Է����������жϣ�D��Ũ����������������Ҵ�����������Ӧ����E��D�к���2���Ȼ�����BΪBrCH2C(CH3)=CHCH2Br��CΪHOCH2C(CH3)=CHCH2OH��DΪHOOCC(CH3)=CHCOOH��EΪCH3CH2OOCC(CH3)=CHCOOCH2CH3��������֪��Ϣ��֪��F�Ľṹ��ʽΪ
��Bͨ��ˮ�ⷴӦ����ԭ�ӱ�ȡ������2���ǻ�����C������D��E����Է����������жϣ�D��Ũ����������������Ҵ�����������Ӧ����E��D�к���2���Ȼ�����BΪBrCH2C(CH3)=CHCH2Br��CΪHOCH2C(CH3)=CHCH2OH��DΪHOOCC(CH3)=CHCOOH��EΪCH3CH2OOCC(CH3)=CHCOOCH2CH3��������֪��Ϣ��֪��F�Ľṹ��ʽΪ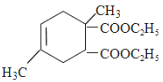 ��
��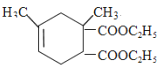 ����G�Ľṹ��ʽ
����G�Ľṹ��ʽ ��
�� ��
��
���������Ϸ�����B���������Ƶ�ˮ��Һ�з���ˮ�ⷴӦ����C��F��Br2�����ӳɷ�Ӧ����G���ʴ�Ϊ��ȡ����Ӧ���ӳɷ�Ӧ��
���������Ϸ�����AΪ![]() ��FΪ
��FΪ ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
�� ��
�� ��
��
��B���������Ƶ�ˮ��Һ�з���ˮ�ⷴӦ����C����B��C����ʽΪ��BrCH2-C(CH3)=CH-CH2Br+2NaOH![]() HOCH2-C(CH3)=CH-CH2OH+2NaBr������D��E����Է����������жϣ�D��Ũ����������������Ҵ�����������Ӧ����E����D��E�ķ���ʽΪ��HOOC-C(CH3)=CH-COOH+2C2H5OH
HOCH2-C(CH3)=CH-CH2OH+2NaBr������D��E����Է����������жϣ�D��Ũ����������������Ҵ�����������Ӧ����E����D��E�ķ���ʽΪ��HOOC-C(CH3)=CH-COOH+2C2H5OH![]() C2H5OOC-C(CH3)=CH-COOC2H5+2H2O���ʴ�Ϊ��BrCH2-C(CH3)=CH-CH2Br+2NaOH
C2H5OOC-C(CH3)=CH-COOC2H5+2H2O���ʴ�Ϊ��BrCH2-C(CH3)=CH-CH2Br+2NaOH![]() HOCH2-C(CH3)=CH-CH2OH+2NaBr��HOOC-C(CH3)=CH-COOH+2C2H5OH
HOCH2-C(CH3)=CH-CH2OH+2NaBr��HOOC-C(CH3)=CH-COOH+2C2H5OH![]() C2H5OOC-C(CH3)=CH-COOC2H5+2H2O��
C2H5OOC-C(CH3)=CH-COOC2H5+2H2O��
��G ������������Һ��Ӧ�ķ���ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����ʵķ�����ж��ַ��������ж��������������ͼ��
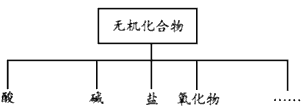
��1����ͼ��ʾ�����ʷ����������____________________��
��2����K��Na��H��O��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��Тڡ��ܡ����档
������� | �� | �� | �� | ������ | �⻯�� |
��ѧʽ | ��HNO3 ��_______ | ��NaOH ��_______ | ��Na2SO4 ��_______ | ��CO2 ��SO3 | ��NH3 |
��3��д�����������Ģ���Һ��Ӧ�����ӷ���ʽ_______________________��
��4��д���������Һ��Ӧ�Ļ�ѧ����ʽ______________________��
��5����ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݡ����ø�Ũ��������480 mL 1 mol�� L��1��ϡ���ᡣ
�ɹ�ѡ�õ������У��ٽ�ͷ�ιܢ���ƿ���ձ�����������ҩ�ע���Ͳ��������ƽ��

��ش��������⣺
a������������ʵ���Ũ��Ϊ __________ mol�� L��1��
b������ϡ����ʱ����ȱ�ٵ������� ______________ (д��������)��
c�������㣬����480mL 1mol�� L��1��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ_________mL��
d���������Ƶ�ϡ������вⶨ��������Ũ�ȴ���1 mol�� L��1�����ƹ��������и�������������������ԭ���� ___________��
A������ʱ����������ƿ�̶��߽��ж��� ��
B����ϡ�ͺ��ϡ��������ת������ƿ�����žͽ����Ժ��ʵ�������
C��ת����Һʱ��������������Һ��������ƿ���档
D������ƿ������ˮϴ�Ӻ�δ���������������ˮ ��
E�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ���

