��Ŀ����
����Ŀ���⻯����һ����ɫ���壬������ˮ��ʵ�����Ʊ�KI����IJ������£�
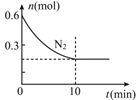
������ͼ��ʾ��������ƿ�м���12.7g��ϸ�ĵ��ʵ��100mL1.5mol/L��KOH��Һ������(��֪��I2��KOH��Ӧ����֮һ��KIO3)
�ڵ���ȫ��Ӧ��Һ©���еĻ����͵��ɼ�1��2.��װ��C��ͨ��������H2S��
�۷�Ӧ��������װ��C�м���ϡH2SO4�ữ��ˮԡ����
����ȴ�����˵�KI����Һ��

(1)����a������_________��
(2)A����ȡH2S����Ļ�ѧ����ʽ___________��
(3)Bװ���б���NaHS��Һ��������_________(�����)��
�ٸ���H2S���� �ڳ�HCl����
(4)D��ʢ�ŵ��Լ���__________��
(5)������з�����Ӧ�����ӷ���ʽ��_____________��
(6)�ɲ�������õ�KI����Һ(��SO42-)���Ʊ�KI�����ʵ�鷽�����߽��������Һ�м���������BaCO3����ֽ��衢���ˡ�ϴ�Ӳ��������Һ��ϴ��Һ�ϲ�������HI��Һ���������ԣ��ڲ��Ͻ������������϶����������ֹͣ���ȣ����������ɣ��õ�KI���壬�����ϣ��õ�KI���������Ϊ________��
���𰸡�Բ����ƿ����ƿ �� FeS +2HCl ==FeCl 2+ H2S�� �� NaOH��Һ 3I2 + 6OH- = IO3-+5 I-+3H2O 24.9g
��������
�Ʊ�KI���رյ��ɼУ�A����ϡ������FeS�Ʊ�H2S���壬Bװ���ɱ���NaHS��Һ��ȥH2S�����е�HCl���ʣ�C��I2��KOH��Һ��Ӧ��3I2+6OH-=5I-+IO3-+3H2O������ȫ��Ӧ���ɼУ���C��ͨ��������H2S��������Ӧ��3H2S+KIO3=3S��+KI+3H2O����װ��C��������Һ��ϡH2SO4�ữ������ˮԡ�ϼ���10min����ȥ��Һ���ܽ��H2S���壬���õ�KI��Һ(��SO42-)������̼�ᱵ��������������ٴ���Һ�Ʊ�KI���壬DΪ������������δ��Ӧ���H2S���壬�ݴ˷������
(1)����װ��ͼ������a������ΪԲ����ƿ���ʴ�Ϊ��Բ����ƿ(����ƿ)��
(2)A�������������ᷴӦ�����������壬��Ӧ�ķ���ʽΪFeS +2HCl=FeCl2+ H2S�����ʴ�Ϊ��FeS +2HCl=FeCl2+ H2S����
(3)�����ӷ���A���Ʊ���H2S�������HCl���壬Bװ���еı���NaHS��Һ���Գ�ȥH2S�����е�HCl���ʣ��ʴ�Ϊ���ڣ�
(4)�������Ⱦ������Dװ��������δ��Ӧ���H2S���壬��ֹ��Ⱦ����������ѡ�ü����Լ�������������Һ���գ��ʴ�Ϊ������������Һ��
(5)������з���I2��KOH��Ӧ����KI��KIO3����Ӧ�����ӷ���ʽΪ��3I2+6OH-=5I-+IO3-+3H2O���ʴ�Ϊ��3I2+6OH-=5I-+IO3-+3H2O��
(6)12.7g�ⵥ�ʵ����ʵ���=![]() 0.05mol��100mL1.5mol/L��KOH��Һ�к�����������0.15mol���õ�KI����Ĺ�����ҪΪ��3I2+6OH-=5I-+IO3-+3H2O��3H2S+KIO3=3S��+KI+3H2O������̼�ᱵ��ȥ��������ӣ����ˡ�ϴ�Ӳ��������HI��Һ���������ԣ��ڲ��Ͻ������������϶��������������Ԫ���غ㣬�ⵥ���еĵ�����ȫ��ת��Ϊ�⻯���еĵ⣬���������еļ�Ԫ������ȫ��ת��Ϊ�⻯���еļأ����KI�����ʵ���=�������ص����ʵ���=0.15mol������=0.15mol��166g/mol=24.9g���ʴ�Ϊ��24.9g��
0.05mol��100mL1.5mol/L��KOH��Һ�к�����������0.15mol���õ�KI����Ĺ�����ҪΪ��3I2+6OH-=5I-+IO3-+3H2O��3H2S+KIO3=3S��+KI+3H2O������̼�ᱵ��ȥ��������ӣ����ˡ�ϴ�Ӳ��������HI��Һ���������ԣ��ڲ��Ͻ������������϶��������������Ԫ���غ㣬�ⵥ���еĵ�����ȫ��ת��Ϊ�⻯���еĵ⣬���������еļ�Ԫ������ȫ��ת��Ϊ�⻯���еļأ����KI�����ʵ���=�������ص����ʵ���=0.15mol������=0.15mol��166g/mol=24.9g���ʴ�Ϊ��24.9g��