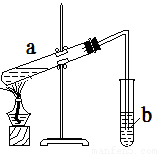
��Ŀ����
| |||||||||||||||||||||||
�𰸣�
������
������
(1) |
�ȼ����Ҵ���Ȼ���ҡ���Թܱ���������Ũ���ᣬ�ټӱ�����(�����Թ�a�����ȼ���Ũ���ᣬ�����0��) |
(2) |
���Թ�a�м��뼸����ʯ(�����Ƭ) |
(3) |
�����ټӿ췴Ӧ����; �����ڼ�ʱ��������������������������ƽ�����������������ķ����ƶ� |
(4) |
�������������������������������ʺ��Ҵ� |
(5) |
b�е�Һ��ֲ㣬�ϲ���������״Һ�� |

��ϰ��ϵ�д�
 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�����Ŀ
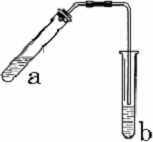
 ����ͼʾװ����ȡ���������������ƾ��Ƶ���ͼ�о�����ȥ������գ�
����ͼʾװ����ȡ���������������ƾ��Ƶ���ͼ�о�����ȥ������գ�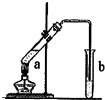 23������ͼʾװ����ȡ�������������������
23������ͼʾװ����ȡ�������������������