��Ŀ����
������ijЩ������������ɵĻ�����ڻ�ѧ�ϳ�Ϊ���ȼ����û�����ڸ����������ܷ����û���Ӧ��Ϊȷ��ij���ȼ���Ʒ������������������ɣ��ֱ��������ʵ�飺
��1����ȡ10.7 g����Ʒ�������м���������NaOH��Һ��������ɵ�����(��״������ͬ)���Ϊa L����Ӧ�Ļ�ѧ����ʽ�� ����Ʒ���������������� ���ú�a�ı���ʽ��ʾ����
��2����ȡͬ��������Ʒ���ڸ�����ʹ��ǡ�÷�Ӧ����a = L���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3������2���з�Ӧ������ȴ�����������ᣬ������ɵ��������Ϊb L���������루1������������������a��b =_______��
�� 2Al + 2NaOH + 2H2O �� 2NaAlO2 + 3H2��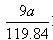 ��0.075a
��0.075a
�� 3.36 2 Al+ Fe2O3 2Fe + Al2O3
2Fe + Al2O3
�� 3��2
����

��ϰ��ϵ�д�
�����Ŀ